Grape reaction product
The grape reaction product (GRP, GRP1 or 2-S-glutathionyl caftaric acid[1]) is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing.[2] It is also found in aged red wines.[3] Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts,[4] especially in white wine production. The product can be recreated in model solutions.[5][6]
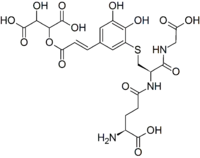 | |
| Names | |
|---|---|
| IUPAC name
2-[(E)-3-[3-[2-[(4-amino-4-carboxybutanoyl)amino]-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-4,5-dihydroxyphenyl]prop-2-enoyl]oxy-3-hydroxybutanedioic acid | |
| Other names
GRP GRP1 2-S-Glutathionyl caftaric acid | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C23H27N3O15S | |
| Molar mass | 617.54 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Determining its concentration in wine is possible by mass spectrometry.[7]
S-Glutathionyl caftaric acid is itself oxidizable.[8] It is not a substrate for grape polyphenol oxidase, but laccase from Botrytis cinerea can use it to form GRP2.[9]
Related molecules
Other related molecules are trans-caffeoyltartrate derivatives like GRP o-quinone[10] and 2,5-di-S-glutathionyl cafteoyl tartrate (GRP2)[11] or adducts with anthocyanidins.[12]

See also
References
- Veronique F. Cheynier; Eugene K. Trousdale; Vernon L. Singleton; Michel J. Salgues; Renee Wylde (1986). "Characterization of 2-S-glutathionyl caftaric acid and its hydrolysis in relation to grape wines". J. Agric. Food Chem. 34 (2): 217–221. doi:10.1021/jf00068a016.
- Caftaric acid in grapes and conversion to a reaction product during processing. V.L. Singleton, J. Zaya, E. Trousdale and M. Salgues, Vitis, 1984, pages 113-120 (article)
- Anis Arnous; Dimitris P. Makris; Panagiotis Kefalas (2001). "Effect of Principal Polyphenolic Components in Relation to Antioxidant Characteristics of Aged Red Wines". J. Agric. Food Chem. 49 (12): 5736–5742. doi:10.1021/jf010827s.
- V.L. Singleton; M. Salgues; J. Zaya; E. Trousdale (1985). "Caftaric Acid Disappearance and Conversion to Products of Enzymic Oxidation in Grape Must and Wine" (PDF). Am. J. Enol. Vitic. 36 (1): 50.
- V. Cheynier; C. Owe; J. Rigaud (1988). "Oxidation of Grape Juice Phenolic Compounds in Model Solutions". Journal of Food Science. 53 (6): 1729–1732. doi:10.1111/j.1365-2621.1988.tb07828.x.
- Veronique Cheynier; Jorge M. Ricardo da Silva (1991). "Oxidation of grape procyanidins in model solutions containing trans-caffeoyltartaric acid and polyphenol oxidase". J. Agric. Food Chem. 39 (6): 1047–1049. doi:10.1021/jf00006a008.
- Straightforward Method To Quantify GSH, GSSG, GRP, and Hydroxycinnamic Acids in Wines by UPLC-MRM-MS. Anna Vallverdú-Queralt, Arnaud Verbaere, Emmanuelle Meudec, Veronique Cheynier and Nicolas Sommerer, J. Agric. Food Chem. 2015, 63, 142−149, doi:10.1021/jf504383g
- Caftaric Acid Disappearance and Conversion to Products of Enzymic Oxidation in Grape Must and Wine. V. L. Singleton, M. Salgues, J. Zaya and E. Trousdale, Am. J. Enol. Vitic, 1985, volume 36, number 1, pages 50-56 'abstract)
- Salgues, M.; Cheynier, V.; Gunata, Z.; Wylde, R. (1986). "Oxidation of Grape Juice 2-S-Glutathionyl Caffeoyl Tartaric Acid by Botrytis cinerea Laccase and Characterization of a New Substance: 2,5-di-S-Glutathionyl Caffeoyl Tartaric Acid". Journal of Food Science. 51 (5): 1191. doi:10.1111/j.1365-2621.1986.tb13081.x.
- "Must browning in relation to the behavior of phenolic compounds during oxidation. Cheynier V., Rigaud J., Souquet J.-M., Duprat F. and Moutounet M., American journal of enology and viticulture, 1990, vol. 41, no4": 346–349, INIST:5402970. Cite journal requires
|journal=(help) - Véronique Cheynier; Jacques Rigaud; Michel Moutounet (1990). "Oxidation kinetics of trans-caffeoyltartrate and its glutathione derivatives in grape musts". Phytochemistry. 29 (6): 1751–1753. doi:10.1016/0031-9422(90)85008-4.
- Petros Kneknopoulos; George K. Skouroumounis; Yoji Hayasaka; Dennis K. Taylor (2011). "New Phenolic Grape Skin Products from Vitis vinifera cv. Pinot Noir". J. Agric. Food Chem. 59 (3): 1005–1011. doi:10.1021/jf103682x. PMID 21214245.