Formylation reaction
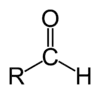
A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (C-CH=O), formamides (N-CH=O), and formate esters (O-CH=O). A reagent that delivers the formyl group is called a formylating agent.[1] A particularly important formylation process is hydroformylation which converts alkenes to the homologated aldehyde. The conversion of benzene to benzaldehyde is the basis of the Gattermann–Koch reaction:
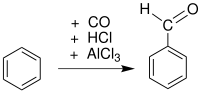
Aromatic formylation
Formylation reactions are a form of electrophilic aromatic substitution and therefore work best when the aromatic starting materials are electron-rich. Phenols are very commonly encountered as they can be readily deprotonated to form phenoxides which are excellent nucleophiles, other electron rich substrates such as mesitylene, pyrrole, or fused aromatic rings can also be expected to react. Benzene will react under aggressive conditions but deactivated rings such as pyridine are difficult to formylate effectively.
Selective ortho-formylation of phenols
Phenol would normally be expected to react to give a mixture of ortho and para products, however many formylation reactions will selectively give only the ortho product (e.g. 2-hydroxybenzaldehyde). This can be explained by strong attractive interactions between the phenoxide and the formylating reagent during the reaction, for example ionic interactions with cationic nitrogen centres in the Vilsmeier–Haack reaction and Duff reaction or coordination to high oxidation metals in the Casiraghi formylation and Rieche formylation (c.f. Kolbe–Schmitt reaction). The direct reaction between phenol and paraformaldehyde is possible via the Casiraghi formylation,[2] all other methods used masked forms of formaldehyde, in part to limit the formation of phenol formaldehyde resins. Aldehydes are strongly deactivating and as such phenols typically only react once, however certain reactions, such as the Duff reaction, can give double addition.[3]
Other substrates
Formylation can be applied to other aromatic rings. As it generally begins with nucleophilic attack by the aromatic group, the electron density of the ring is an important factor. Some aromatic compounds, such as pyrrole, are known to formylate regioselectively.[4]
Formylation of benzene rings can be achieved via the Gattermann reaction and Gattermann-Koch reaction. These involve strong acid catalysis and proceed in a manner similar to the Friedel–Crafts reaction.
List of aromatic formylation reactions
- Dimethylformamide and phosphorus oxychloride in the Vilsmeier-Haack reaction.[5]
- Hexamethylenetetramine in the Duff reaction and the Sommelet reaction
- Carbon monoxide and hydrochloric acid in the Gattermann-Koch reaction
- Cyanides in the Gattermann reaction. This method synthetizes aromatic aldehydes using hydrogen chloride and hydrogen cyanide (or another metallic cyanide as such zinc cyanide) in the presence of Lewis acid catalysts:
- Chloroform in the Reimer-Tiemann reaction
- dichloromethyl methyl ether in Rieche formylation. Formylation of 3-methylamino-1-propanol with formamide (instead of ethyl chloroformate) as a protecting group in the preparation of Protriptyline. Formamide was also used (instead of formic acid) in the synthesis of primidone.
Aliphatic formylation
Hydroformylation of alkenes is the most important method of aliphatic formylation, however it is largely restricted to an industrial setting due to the high temperatures and pressures involved. Several specialty methods exist for laboratory-scale synthesis, including the Sommelet reaction, Bouveault aldehyde synthesis or Bodroux–Chichibabin aldehyde synthesis.
See also
References
- Olah, G. A.; Ohannesian, L.; Arvanaghi, M. (1987). "Formylating agents". Chem. Rev. 87: 671–686. doi:10.1021/cr00080a001.CS1 maint: uses authors parameter (link)
- Casiraghi, Giovanni; Casnati, Giuseppe; Puglia, Giuseppe; Sartori, Giovanni; Terenghi, Giuliana (1980). "Selective reactions between phenols and formaldehyde. A novel route to salicylaldehydes". Journal of the Chemical Society, Perkin Transactions 1: 1862. doi:10.1039/P19800001862.
- Lindoy, Leonard F. (July 1998). "Mono- and Diformylation of 4-Substituted Phenols: A New Application of the Duff Reaction". Synthesis. 1998 (07): 1029–1032. doi:10.1055/s-1998-2110.
- Warashina, Takuya; Matsuura, Daisuke; Sengoku, Tetsuya; Takahashi, Masaki; Yoda, Hidemi; Kimura, Yoshikazu (16 October 2018). "Regioselective Formylation of Pyrrole-2-Carboxylate: Crystalline Vilsmeier Reagent vs Dichloromethyl Alkyl Ether". Organic Process Research & Development. doi:10.1021/acs.oprd.8b00233.
- Ding, S.; Jiao, N. (2012). "N,N-Dimethylformamide: A Multipurpose Building Block". Angew. Chem. Int. Ed. 51: 9226–9237. doi:10.1002/anie.201200859.CS1 maint: uses authors parameter (link)