Guvacine
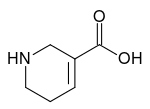
Guvacine is an pyridine alkaloid in areca nuts.[1] It is the N-demethylated derivative of arecaidine and the product of ester hydrolysis of guvacoline, both of which are also found in areca nuts as well. It is also an inhibitor of gamma-aminobutyric acid. Lime is said to hydrolyse guvacoline to guvacine.[2]
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,6-Tetrahydropyridine-5-carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H9NO2 | |
| Molar mass | 127.143 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Voigt, V; Laug, L; Zebisch, K; Thondorf, I; Markwardt, F; Brandsch, M (2013). "Transport of the areca nut alkaloid arecaidine by the human proton-coupled amino acid transporter 1 (hPAT1)". The Journal of Pharmacy and Pharmacology. 65 (4): 582–90. doi:10.1111/jphp.12006. PMID 23488788.
- Johnston, G. A. R.; Krogsgaard-Larsen, P.; Stephanson, A. (1975). "Betel nut constituents as inhibitors of γ-aminobutyric acid uptake". Nature. 258 (5536): 627–628. doi:10.1038/258627a0. ISSN 0028-0836.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.