3-Mercaptopropionic acid
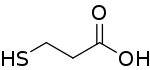
3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulfide to acrylic acid.
 | |
| Names | |
|---|---|
| IUPAC name
3-Sulfanylpropanoic acid | |
| Other names
3-MPA; 3-Mercaptopropanoic acid; β-Mercaptopropionic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 773807 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.216 |
| EC Number |
|
| 101294 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6O2S | |
| Molar mass | 106.14 g·mol−1 |
| Density | 1.218 |
| Melting point | 16.9 °C (62.4 °F; 290.0 K) |
| Boiling point | 111 °C (232 °F; 384 K) |
| soluble | |
| Solubility | ether benzene alcohol water |
| Acidity (pKa) | 4.34 |
Refractive index (nD) |
1.4911 at 21 °C |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H290, H301, H314, H318, H332 | |
| P234, P260, P261, P264, P270, P271, P280, P301+310, P301+330+331, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P330, P363, P390, P404, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions and uses
It is competitive inhibitor of glutamate decarboxylase, and therefore acts as a convulsant. It has higher potency and faster onset of action compared to allylglycine.[1]
It is used to prepare hydrophilic gold nanoparticles, exploiting the affinity of gold for sulfur ligands.[2]
See also
- Allylglycine
- Thiolactic acid (2-mercaptopropionic acid)
References
- Horton, R. W; Meldrum, B. S (1973). "Seizures induced by allylglycine, 3-mercaptopropionic acid and 4-deoxypyridoxine in mice and photosensitive baboons, and different modes of inhibition of cerebral glutamic acid decarboxylase". British Journal of Pharmacology. 49 (1): 52–63. doi:10.1111/j.1476-5381.1973.tb08267.x. PMC 1776427. PMID 4207045.
- Subramanian, Vaidyanathan; Wolf, Eduardo E.; Kamat, Prashant V. (2004). "Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration". Journal of the American Chemical Society. 126: 4943–4950. doi:10.1021/ja0315199.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.