Human genetic enhancement
Human genetic enhancement or human genetic engineering refers to human enhancement by means of a genetic modification. This could be done in order to cure diseases (gene therapy), prevent the possibility of getting a particular disease[1] (similarly to vaccins), to improve athlete performance in sporting events (gene doping), or to change physical appearance, metabolism, and even improve physical capabilities and mental faculties such as memory and intelligence. These genetic enhancements may or may not be done in such a way that the change is heritable (which has raised concerns within the scientific community.[2]
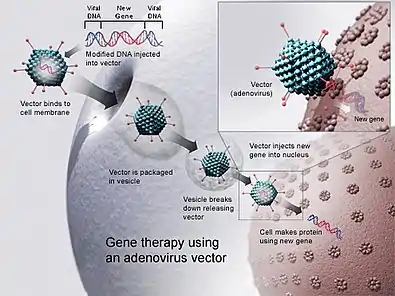
Gene therapy
Genetic modification in order to cure genetic diseases is referred to as gene therapy. Many such gene therapies are available, made it through all phases of clinical research and are approved by the FDA. Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in phase I.[3] As of 2017, Spark Therapeutics' Luxturna (RPE65 mutation-induced blindness) and Novartis' Kymriah (Chimeric antigen receptor T cell therapy) are the FDA's first approved gene therapies to enter the market. Since that time, drugs such as Novartis' Zolgensma and Alnylam's Patisiran have also received FDA approval, in addition to other companies' gene therapy drugs. Most of these approaches utilize adeno-associated viruses (AAVs) and lentiviruses for performing gene insertions, in vivo and ex vivo, respectively. ASO / siRNA approaches such as those conducted by Alnylam and Ionis Pharmaceuticals require non-viral delivery systems, and utilize alternative mechanisms for trafficking to liver cells by way of GalNAc transporters.
Disease prevention
Some people are immunocompromised and their bodies are hence much less capable of fending off and defeating diseases (i.e. influenza, ...). In some cases this is due to genetic flaws or even genetic diseases such as SCID. Some gene therapies have already been developed or are being developed to correct these genetic flaws/diseases, hereby making these people less susceptible to catching additional diseases (i.e. influenza, ...).[4]
In November 2018, Lulu and Nana were created.[5] By using clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9, a gene editing technique, they disabled a gene called CCR5 in the embryos, aiming to close the protein doorway that allows HIV to enter a cell and make the subjects immune to the HIV virus.
Gene doping
Athletes might adopt gene therapy technologies to improve their performance.[6] Gene doping is not known to occur, but multiple gene therapies may have such effects. Kayser et al. argue that gene doping could level the playing field if all athletes receive equal access. Critics claim that any therapeutic intervention for non-therapeutic/enhancement purposes compromises the ethical foundations of medicine and sports.[7]
Other uses
Other hypothetical gene therapies could include changes to physical appearance, metabolism, mental faculties such as memory and intelligence.
Physical appearance
Some congenital disorders (such as those affecting the muscoskeletal system) may affect physical appearance, and in some cases may also cause physical discomfort. Modifying the genes causing these congenital diseases (on those diagnosed to have mutations of the gene known to cause these diseases) may prevent this.
Also changes in the mystatin gene[8] may alter appearance.
Behavior
Behavior may also be modified by genetic intervention.[9] Some people may be aggressive, selfish, ... and may not be able to function well in society. There is currently research ongoing on genes that are or may be (in part) responsible for selfishness (i.e. ruthlessness gene, aggression (i.e. warrior gene), altruism (i.e. OXTR, CD38, COMT, DRD4, DRD5, IGF2, GABRB2[10])
There is some research going on on the hypothetical treatment of psychiatric disorders by means of gene therapy. It is assumed that, with gene-transfer techniques, it is possible (in experimental settings using animal models) to alter CNS gene expression and thereby the intrinsic generation of molecules involved in neural plasticity and neural regeneration, and thereby modifying ultimately behaviour.[11]
In recent years, it was possible to modify ethanol intake in animal models. Specifically, this was done by targeting the expression of the aldehyde dehydrogenase gene (ALDH2), lead to a significantly altered alcohol-drinking behaviour.[12] Reduction of p11, a serotonin receptor binding protein, in the nucleus accumbens led to depression-like behaviour in rodents, while restoration of the p11 gene expression in this anatomical area reversed this behaviour.[13]
Recently, it was also shown that the gene transfer of CBP (CREB (c-AMP response element binding protein) binding protein) improves cognitive deficits in an animal model of Alzheimer’s dementia via increasing the expression of BDNF (brain-derived neurotrophic factor).[14] The same authors were also able to show in this study that accumulation of amyloid-β (Aβ) interfered with CREB activity which is physiologically involved in memory formation.
In another study, it was shown that Aβ deposition and plaque formation can be reduced by sustained expression of the neprilysin (an endopeptidase) gene which also led to improvements on the behavioural (i.e. cognitive) level.[15]
Similarly, the intracerebral gene transfer of ECE (endothelin-converting enzyme) via a virus vector stereotactically injected in the right anterior cortex and hippocampus, has also shown to reduce Aβ deposits in a transgenic mouse model of Alzeimer’s dementia.[16]
There is also research going on on genoeconomics, a protoscience that is based on the idea that a person's financial behavior could be traced to their DNA and that genes are related to economic behavior. As of 2015, the results have been inconclusive. Some minor correlations have been identified.[17][18]
See also
- Crossbreeding
- Directed evolution (transhumanism)
- Designer baby
- Epigenetics
- Genetic screening: allows detecting personal genetic weaknesses to be addressed
- Procreative beneficence
- New eugenics
- Life extension
References
- Veit, W. (2018). Procreative Beneficence and Genetic Enhancement – KRITERION – Journal of Philosophy 32(1):75-92. https://doi.org/10.13140/RG.2.2.11026.89289
- "1990 The Declaration of Inuyama". 5 August 2001. Archived from the original on 5 August 2001.CS1 maint: bot: original URL status unknown (link)
- Gene Therapy Clinical Trials Worldwide Database. The Journal of Gene Medicine. Wiley (June 2016)
- Garcia-Perez, Laura; van Eggermond, Marja; van Roon, Lieke; Vloemans, Sandra A.; Cordes, Martijn; Schambach, Axel; Rothe, Michael; Berghuis, Dagmar; Lagresle-Peyrou, Chantal; Cavazzana, Marina; Zhang, Fang; Thrasher, Adrian J.; Salvatori, Daniela; Meij, Pauline; Villa, Anna; Van Dongen, Jacques J.M.; Zwaginga, Jaap-Jan; van der Burg, Mirjam; Gaspar, H. Bobby; Lankester, Arjan; Staal, Frank J.T.; Pike-Overzet, Karin (June 2020). "Successful Preclinical Development of Gene Therapy for Recombinase-Activating Gene-1-Deficient SCID". Molecular Therapy - Methods & Clinical Development. 17: 666–682. doi:10.1016/j.omtm.2020.03.016. PMID 32322605. S2CID 216061532.
- Ma, Hong; Marti-Gutierrez, Nuria; Park, Sang-Wook; Wu, Jun; Lee, Yeonmi; Suzuki, Keiichiro; Koski, Amy; Ji, Dongmei; Hayama, Tomonari; Ahmed, Riffat; Darby, Hayley; Van Dyken, Crystal; Li, Ying; Kang, Eunju; Park, A.-Reum; Kim, Daesik; Kim, Sang-Tae; Gong, Jianhui; Gu, Ying; Xu, Xun; Battaglia, David; Krieg, Sacha A.; Lee, David M.; Wu, Diana H.; Wolf, Don P.; Heitner, Stephen B.; Belmonte, Juan Carlos Izpisua; Amato, Paula; Kim, Jin-Soo; Kaul, Sanjiv; Mitalipov, Shoukhrat (August 2017). "Correction of a pathogenic gene mutation in human embryos". Nature. 548 (7668): 413–419. Bibcode:2017Natur.548..413M. doi:10.1038/nature23305. PMID 28783728. S2CID 205258702.
- "WADA Gene Doping". WADA. Archived from the original on 21 November 2009. Retrieved 27 September 2013.
- Kayser, Bengt; Mauron, Alexandre; Miah, Andy (December 2007). "Current anti-doping policy: a critical appraisal". BMC Medical Ethics. 8 (1): 2. doi:10.1186/1472-6939-8-2. PMC 1851967. PMID 17394662.
- Gavish, B.; Gratton, E.; Hardy, C. J. (1 February 1983). "Adiabatic compressibility of globular proteins". Proceedings of the National Academy of Sciences. 80 (3): 750–754. Bibcode:1983PNAS...80..750G. doi:10.1073/pnas.80.3.750. PMC 393457. PMID 6572366.
- Lupton, ML (1994). "Behaviour modification by genetic intervention--the law's response". Medicine and Law. 13 (5–6): 417–31. PMID 7845173.
- Thompson, Graham J.; Hurd, Peter L.; Crespi, Bernard J. (23 December 2013). "Genes underlying altruism". Biology Letters. 9 (6): 20130395. doi:10.1098/rsbl.2013.0395. PMC 3871336. PMID 24132092.
- Thome, Johannes; Hässler, Frank; Zachariou, Vanna (September 2011). "Gene therapy for psychiatric disorders". The World Journal of Biological Psychiatry. 12 (sup1): 16–18. doi:10.3109/15622975.2011.601927. PMC 3394098. PMID 21905989.
- Ocaranza, Paula; Quintanilla, María Elena; Tampier, Lutske; Karahanian, Eduardo; Sapag, Amalia; Israel, Yedy (19 October 2007). "Gene Therapy Reduces Ethanol Intake in an Animal Model of Alcohol Dependence". Alcoholism: Clinical and Experimental Research. 32 (1): 52–57. doi:10.1111/j.1530-0277.2007.00553.x. hdl:10533/139024. PMID 18070247.
- Alexander, Brian; Warner-Schmidt, Jennifer; Eriksson, Therese M.; Tamminga, Carol; Arango-Lievano, Margarita; Ghose, Subroto; Vernov, Mary; Stavarache, Mihaela; Musatov, Sergei; Flajolet, Marc; Svenningsson, Per; Greengard, Paul; Kaplitt, Michael G. (20 October 2010). "Reversal of Depressed Behaviors by p11 Gene Therapy in the Nucleus Accumbens". Science Translational Medicine. 2 (54): 54ra76. doi:10.1126/scitranslmed.3001079. PMC 3026098. PMID 20962330.
- Caccamo, Antonella; Majumder, Smita; Richardson, Arlan; Strong, Randy; Oddo, Salvatore (23 April 2010). "Molecular Interplay between Mammalian Target of Rapamycin (mTOR), Amyloid-β, and Tau". The Journal of Biological Chemistry. 285 (17): 13107–13120. doi:10.1074/jbc.M110.100420. PMC 2857107. PMID 20178983.
- Spencer, Brian; Marr, Robert A; Rockenstein, Edward; Crews, Leslie; Adame, Anthony; Potkar, Rewati; Patrick, Christina; Gage, Fred H; Verma, Inder M; Masliah, Eliezer (12 November 2008). "Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice". BMC Neuroscience. 9: 109. doi:10.1186/1471-2202-9-109. PMC 2596170. PMID 19014502.
- Carty, Niki C; Nash, Kevin; Lee, Daniel; Mercer, Mary; Gottschall, Paul E; Meyers, Craig; Muzyczka, Nicholas; Gordon, Marcia N; Morgan, Dave (September 2008). "Adeno-associated Viral (AAV) Serotype 5 Vector Mediated Gene Delivery of Endothelin-converting Enzyme Reduces Aβ Deposits in APP + PS1 Transgenic Mice". Molecular Therapy. 16 (9): 1580–1586. doi:10.1038/mt.2008.148. PMC 2706523. PMID 18665160. ProQuest 1792610385.
- Neyfakh, Leon (May 13, 2012). "In search of the money gene". The Boston Globe.
- Entine, Jon (14 October 2012). "Genoeconomics: Is Our Financial Future In Our Chromosomes?". Science 2.0.