Genetic history of East Asians
The article genetic history of East Asians explains the genetic makeup of the East Asian peoples.
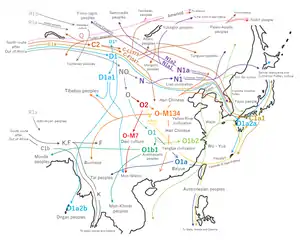
Xiongnu people (ancient)
The Xiongnu, possibly a Turkic, Iranian, Mongolic, Yenisseian or multi-ethnic people, were a confederation[1] of nomadic peoples who, according to ancient Chinese sources, inhabited the eastern Asian Steppe from the 3rd century BC to the late 1st century AD. Chinese sources report that Modu Chanyu, the supreme leader after 209 BC, founded the Xiongnu Empire.[2]
A majority (89%) of the Xiongnu sequences can be classified as belonging to Asian haplogroups, and nearly 11% belong to European haplogroups.[3]
Paternal lineages
Over the past decade, Chinese archaeologists have published several reviews regarding the results of excavations in Xinjiang. They imply the genetic composition of Xiongnu's supreme ruling class. Particularly interesting are the tombs in the cemetery at Heigouliang, Xinjiang (the Black Gouliang cemetery, also known as the summer palace of the Xiongnu king), east of the Barkol basin, near the city of Hami. By typing results of DNA samples during the excavation of one of the tombs, it was determined that of the 12 men: 6 Q1a* (not Q1a1-M120, not Q1a1b-M25, not Q1a2-M3), 4 Q1b-M378, 2 Q* (not Q1a, not Q1b: unable to determine subclades):[4]
In a paper (Lihongjie 2012), the author analyzed the Y-DNAs of the ancient male samples from the 2nd or 1st century BCE cemetery at Heigouliang in Xinjiang – which is also believed to be the site of a summer palace for Xiongnu kings – which is east of the Barkol basin and near the city of Hami. The Y-DNA of 12 men excavated from the site belonged to Q-MEH2 (Q1a) or Q-M378 (Q1b). The Q-M378 men among them were regarded as hosts of the tombs; half of the Q-MEH2 men appeared to be hosts and the other half as sacrificial victims.
Xianbei people (ancient)
The origins of the Xianbei are unclear. Chinese anthropologist Zhu Hong and Zhang Quanchao studied Xianbei crania from several sites of Inner Mongolia and noticed that anthropological features of studied Xianbei crania show that the racial type is closely related to the modern East-Asian Mongoloids, and some physical characteristics of those skulls are closer to modern Mongols, Manchu and Han Chinese.[5]
Maternal lineages
Genetic studies published in 2006 and 2015 revealed that the mitochondrial haplogroups of Xianbei remains were of East Asian origin. According to Zhou (2006) the haplogroup frequencies of the Tuoba Xianbei were 43.75% haplogroup D, 31.25% haplogroup C, 12.5% haplogroup B, 6.25% haplogroup A and 6.25% "other."[6]
Zhou (2014) obtained mitochondrial DNA analysis from 17 Tuoba Xianbei, which indicated that these specimens were, similarly, completely East Asian in their maternal origins, belonging to haplogroups D, C, B, A and haplogroup G.[7]
The research also found a relation between Xianbei individuals with modern Oroqen, Ewenki and Outer Mongolian people. Especially Tungusic Oroqen show close relation to Xianbei.[8]
Genetic history of Han Chinese
A 2018 study calculated pairwise FST (a measure of genetic difference) based on genome-wide SNPs, among the Han Chinese (Northern Han from Beijing and Southern Han from Hunan and Fujian provinces), Japanese and Korean populations sampled. It found that the smallest FST value was between North Han Chinese (CHB) and South Han Chinese (CHS) (FST[CHB-CHS] = 0.0014), while CHB and Korean (KOR) (FST[CHB-KOR] = 0.0026) and between KOR and Japanese (JPT) (FST[JPT-KOR] = 0.0033). Generally, pairwise FST between Han Chinese, Japanese and Korean (0.0026~ 0.0090) are greater than that within Han Chinese (0.0014). These results suggested Han Chinese, Japanese and Korean are different in terms of genetic make-up, and the difference among the three groups are much larger than that between northern and southern Han Chinese.[9]
Another study shows that the northern and southern Han Chinese are genetically closest to each other and it finds that the genetic characteristics of present-day northern Han Chinese was already formed as early as three-thousand years ago in the Central Plain area.[10]
A recent genetic study on the remains of people (~4000 years BP) from the Mogou site in the Gansu-Qinghai (or Ganqing) region of China revealed more information on the genetic contributions of these ancient Di-Qiang people to the ancestors of the Northern Han. It was deduced that 3300–3800 years ago some Mogou people had merged into the ancestral Han population, resulting in the Mogou people being similar to some northern Han in sharing up to ~33% paternal (O3a) and ~70% maternal (D, A, F, M10) haplogroups. The mixture rate was possibly 13-18%.[11]
The estimated contribution of northern Han to southern Han is substantial in both paternal and maternal lineages and a geographic cline exists for mtDNA. As a result, the northern Han are the primary contributors to the gene pool of the southern Han. However, it is noteworthy that the expansion process was dominated by males, as is shown by a greater contribution to the Y-chromosome than the mtDNA from northern Han to southern Han. These genetic observations are in line with historical records of continuous and large migratory waves of northern China inhabitants escaping warfare and famine, to southern China. Aside from these large migratory waves, other smaller southward migrations occurred during almost all periods in the past two millennia.[12] A study by the Chinese Academy of Sciences into the gene frequency data of Han subpopulations and ethnic minorities in China, showed that Han subpopulations in different regions are also genetically quite close to the local ethnic minorities, meaning that in many cases, blood of ethnic minorities had mixed into Han, while at the same time, the blood of Han had also mixed into the local ethnic minorities.[13] A study on Armenian admixture in varied populations found 3.9% Armenian-like DNA in some northern Chinese Han.[14]
A recent, and to date the most extensive, genome-wide association study of the Han population, shows that geographic-genetic stratification from north to south has occurred and centrally placed populations act as the conduit for outlying ones.[15] Ultimately, with the exception in some ethnolinguistic branches of the Han Chinese, such as Pinghua, there is "coherent genetic structure" (homogeneity) in all Han Chinese.[16]
While Northern Vietnam Kinh people assimilated Han Chinese immigrants into their population, have a sinicized culture and carry the patrilineal Han Chinese O-M7 haplogroup, Cham people carry the patrilineal R-M17 haplogroup of South Asian Indian origin from South Asian merchants spreading Hinduism to Champa and marrying Cham females since Chams have no matrilineal South Asian mtdna and this fits with the matrilocal structure of Cham families.[17] Analysis of Vietnamese Kinh people's genetics show that within the last 800 years there was mixture between a Malay like southern Asian and a Chinese ancestral component that happens to fit the time period in which Kinh expanded south from their Red river delta homeland in Nam tiến which also matches the event 700 years ago when the Cham population suffered massive losses. Japanese and Middle Eastern merchants as well as other foreigners have travelled to the coast of Vietnam for more than 2,000 years and left genetic traces among Vietnamese due to its location on the Silk Roads and commercial paths. Vietnamese ethnicities descend from groups that migrated to SEA islands and the SEA mainland from the region in China south of the river Yangtze.[18] With the exception of Cham who are Austronesian speaking and Mang who are Austroasiatic speaking, the southern Han Chinese and all other ethnic groups in Vietnam share ancestry.[19]
Paternal lineages
Y-chromosome haplogroup O2-M122 is a common DNA marker in Han Chinese, as it appeared in China in prehistoric times. It is found in more than 50% of Chinese males, and ranging up to over 80% in certain regional subgroups of the Han ethnicity.[20] Other Y-DNA haplogroups that have been found with notable frequency in samples of Han Chinese include O-P203 (15/165 = 9.1%, 47/361 = 13.0%), C-M217 (10/168 = 6.0%, 27/361 = 7.5%, 187/1730 = 10.8%, 20/166 = 12.0%), N-M231 (6/166 = 3.6%, 18/361 = 5.0%, 117/1729 = 6.8%, 17/165 = 10.3%), O-M268(xM95, M176) (54/1147 = 4.7%,[21] 8/168 = 4.8%, 23/361 = 6.4%, 12/166 = 7.2%), and Q-M242 (2/168 = 1.2%, 49/1729 = 2.8%, 12/361 = 3.3%, 48/1147 = 4.2%[21]). However, the mitochondrial DNA (mtDNA) of Han Chinese increases in diversity as one looks from northern to southern China, which suggests that male migrants from northern China married with women from local peoples after arriving in modern-day Guangdong, Fujian, and other regions of southern China.[12][22] Despite this, tests comparing the genetic profiles of northern Han, southern Han and southern natives determined that haplogroups O1b-M110, O2a1-M88 and O3d-M7, which are prevalent in southern natives, were only observed in some southern Han (4% on average), but not in northern Han. Therefore, this proves that the male contribution of southern natives in southern Han is limited, assuming that the frequency distribution of Y lineages in southern natives represents that before the expansion of Han culture that started two-thousand years ago.[12][23] In contrast, there are consistent strong genetic similarities in the Y chromosome haplogroup distribution between the southern and northern Chinese population, and the result of principal component analysis indicates almost all Han populations form a tight cluster in their Y chromosome. However, other research has also shown that the paternal lineages Y-DNA O-M119,[24] O-P201,[25] O-P203[25] and O-M95[26] are found in both southern Han Chinese and South Chinese minorities, but more commonly in the latter. In fact, these paternal markers are in turn less frequent in northern Han Chinese.[27][28]
Maternal lineages
The mitochondrial-DNA haplogroups of the Han Chinese can be classified into the northern East Asian-dominating haplogroups, including A, C, D, G, M8, M9, and Z, and the southern East Asian-dominating haplogroups, including B, F, M7, N*, and R.[12] These haplogroups account for 52.7% and 33.85% of those in the Northern Han, respectively. Among these haplogroups, D, B, F, and A were predominant in the Northern Han, with frequencies of 25.77%, 11.54%, 11.54%, and 8.08%, respectively. However, in the Southern Han, the northern and southern East Asian-dominating haplogroups accounted for 35.62% and 51.91%, respectively. The frequencies of haplogroups D, B, F, and A reached 15.68%, 20.85%, 16.29%, and 5.63%, respectively.[10][29][30][31][32]
Genetic history of Manchus and Daurs
Paternal lineages
Haplogroup C3b2b1*-M401(xF5483)[33][34][35] has been identified as a possible marker of the Aisin Gioro and is found in ten different ethnic minorities in northern China, but completely absent from Han Chinese.[36][37][35]
Genetic testing also showed that the haplogroup C3b1a3a2-F8951 of the Aisin Gioro family came to southeastern Manchuria after migrating from their place of origin in the Amur river's middle reaches, originating from ancestors related to Daurs in the Transbaikal area. The Tungusic speaking peoples mostly have C3c-M48 as their subclade of C3 which drastically differs from the C3b1a3a2-F8951 haplogroup of the Aisin Gioro which originates from Mongolic speaking populations like the Daur. Jurchen (Manchus) are a Tungusic people. The Mongol Genghis Khan's haplogroup C3b1a3a1-F3796 (C3*-Star Cluster) is a fraternal "brother" branch of C3b1a3a2-F8951 haplogroup of the Aisin Gioro.[38]
A genetic test was conducted on seven men who claimed Aisin Gioro descent with three of them showing documented genealogical information of all their ancestors up to Nurhaci. Three of them turned out to share the C3b2b1*-M401(xF5483) haplogroup, out of them, two of them were the ones who provided their documented family trees. The other four tested were unrelated.[39] The Daur Ao clan carries the unique haplogroup subclade C2b1a3a2-F8951, the same haplogroup as Aisin Gioro and both Ao and Aisin Gioro only diverged merely a couple of centuries ago from a shared common ancestor. Other members of the Ao clan carry haplogroups like N1c-M178, C2a1b-F845, C2b1a3a1-F3796 and C2b1a2-M48. People from northeast China, the Daur Ao clan and Aisin Gioro clan are the main carriers of haplogroup C2b1a3a2-F8951. The Mongolic C2*-Star Cluster (C2b1a3a1-F3796) haplogroup is a fraternal branch to Aisin Gioro's C2b1a3a2-F8951 haplogroup.[40]
Genetic history of Japanese
Jōmon people
Jōmon people is the generic name of people who lived in the Japanese archipelago during the Jōmon period. Today most Japanese historians believe that the Jomon people were not one homogeneous people but were at least two or three distinct groups.[41]
Recent full genome analyses in 2020 by Boer et al. 2020 and Yang et al. 2020, reveals some further information regarding the origin of the Jōmon peoples. They were found to have largely formed from a Paleolithic Siberian population and an East Asian related population.[42][43]
Yayoi people
The Yayoi people were migrants to the Japanese archipelago from Asia (Korea or China) during the Yayoi period (1000 BCE–300 CE) and Kofun period (250–538 CE). They are seen as direct ancestors of the modern Yamato people, the majority of Japanese and of the Ryukyuan people. It is estimated that modern Japanese share in average about 90% of their genome with the Yayoi.[44]
Paternal lineages
It is estimated that Yayoi people mainly belonged to Haplogroup O-M176 (O1b2) (found in ~32% of present-day Japanese males), Haplogroup O-M122 (O2, formerly O3, today ~20%), Haplogroup O-K18 (O1b1, today ~1%), and Haplogroup O-M119 (O1a, today ~1%), which are typical for East and Southeast Asians.[45][46] Mitsuru Sakitani suggests that haplogroup O1b2, which is common in today's Japanese, Koreans, and some Manchu, and O1a are one of the carriers of Yangtze civilization. As the Yangtze civilization declined several tribes crossed westward and northerly, to the Shandong peninsula, the Korean Peninsula and the Japanese archipelago.[47] One study calls haplogroup O1b1 as a major Austroasiatic paternal lineage and the haplogroup O1b2 (of Koreans and Japanese) as a "para-Austroasiatic" paternal lineage.[48]
Maternal lineages
A recent study confirms that modern Japanese are predominantly descendants of the Yayoi. The mitochondrial chromosomes of modern Japanese are nearly identical with the Yayoi and differ significantly from the Jomon population (see below).[49]
Paternal lineages
The main paternal haplogroups of modern Yamato Japanese are Haplogroup D-M55 (today ~33%, with the frequency in various samples ranging from 18/70 = 25.7% in a sample from Tokushima Prefecture[50] to 24/59 = 40.7% in a sample of Japanese male volunteers[51] and 11/27 = 40.7% in a sample from Aomori Prefecture[52]), Haplogroup O-M176 (O1b2) (today ~32%, range 37/142 = 26.1% in a sample of Japanese[53] to 35/97 = 36.1% in a sample from Western Japan[45]), Haplogroup O-M122 (O2, formerly O3) (today ~20%, ranging from 4/59 = 6.8% in a sample of Japanese volunteers[51] and 11/102 = 10.8% in a sample of adults from Fukuoka[54] to 38/157 = 24.2% in a sample of Japanese[55] and 14/53 = 26.4% in a sample from Kyushu[50]), Haplogroup C-M217 (C2, today ~6%, ranging from 0/26 = 0.0% in a sample from Aomori,[50] 1/61 = 1.6% in a sample from Shizuoka,[50] 1/47 = 2.1% in a sample of Japanese,[56] and 3/137 = 2.2% in a sample from the Kantō region[45] to 15/206 = 7.3% in a sample from Sapporo,[54] 18/241 = 7.5% in a sample from Osaka,[54] 4/53 = 7.5% in a sample from Kyushu,[50] and 8/102 = 7.8% in a sample from Fukuoka[54]), and Haplogroup C-M8 (C1a1, today ~5%, ranging from 0/53 = 0.0% in a sample from Kyushu[50] to 7/70 = 10.0% in a sample from Tokushima[50]).[45][46][57][54] Haplogroups N-M231, O-M119, O-K18, and Q-M242 also have been observed with low frequency among present-day Japanese.
A comprehensive study of worldwide Y-DNA diversity (Underhill et al. 2000) included a sample of 23 males from Japan, of whom eight (35%) belonged to haplogroup D-M174, six (26%) belonged to O-M175, five (22%) belonged to O-M122, three (13%) belonged to C-M8 and C-M130, and one (4.3%) belonged to N-M128.[58]
Among 259 males from Japan (70 from Tokushima, 61 from Shizuoka, 53 from Kyūshū, 45 from Okinawa, 26 from Aomori, and 4 Ainus) whose Y-DNA has been examined in a 2005 study by Michael F. Hammer, ninety (34.7%) belong to haplogroup D-M55, eighty-two (31.7%) belong to haplogroup O-P31 (including 22% O-47z, 7.7% O-M176(x47z), and 1.9% O-M95(xM111)), fifty-two (20.1%) belong to haplogroup O-M122, fourteen (5.4%) belong to haplogroup C-M8, ten (3.9%) belong to haplogroup NO-M214(xO-M175) (including 2.3% NO-M214(xO-M175, N-LLY22g), 1.2% haplogroup N-LLY22g(xM128, P43, M178), and 0.4% haplogroup N-M178), and eight (3.1%) belong to haplogroup C-M217 (including 1.9% haplogroup C-M217(xM86) and 1.2% haplogroup C-M86).[59]
The patrilines belonging to D-P37.1 were found in all the Japanese samples, but were more frequently found in the Ainu (75.0%) and Okinawa (55.6%) samples and less frequently found in the Tokushima (25.7%) and Kyūshū samples (26.4%).[59] Haplogroups O-M175 and C-M8 were not found in the small Ainu sample of four individuals, and C-M217 was not found in the Okinawa sample of 45 individuals.[59] Haplogroup N was detected in samples of Japanese from Aomori (2/26 N-LLY22g(xM128, P43, M178)), Shizuoka (1/61 N-LLY22g(xM128, P43, M178)), and Tokushima (1/70 N-M178), but was not found in the Kyūshū, Okinawa, or Ainu samples.[59] This study, and others, report that Y-chromosome patrilines crossed from the Asian mainland into the Japanese archipelago, and continue to make up a large proportion of the Japanese male lineage.[60] If focusing haplogroup O-P31 in those researches, the patrilines derived from its subclade O-SRY465 are frequently found in both Japanese (mean 32%,[61] with frequency in various samples ranging from 26%[62][63] to 36%[64]) and Koreans (mean 30%,[65] with frequency in various samples ranging from 19%[62][66] to 40%[64]). According to the research, these patrilines have undergone extensive genetic admixture with the Jōmon period populations previously established in Japan.[59]
A 2007 study by Nonaka et al. reported that among a total of 263 healthy unrelated Japanese male individuals born in 40 of the 47 prefectures of Japan, but especially Tokyo (n=51), Chiba (n=45), Kanagawa (n=14), Saitama (n=13), Shizuoka (n=12), and Nagano (n=11), the frequencies of the D2, O2b, and O3 lineages were 38.8%, 33.5%, and 16.7%, respectively, which constituted approximately 90% of the Japanese population. Haplogroup diversity for the binary polymorphisms was calculated to be 86.3%.[67]
Poznik et al. (2016) have reported that the males in the JPT (Japanese in Tokyo, Japan) sample[68] of the 1000 Genomes Project are 20/56 = 36% D2-M179, 18/56 = 32% O2b-M176, 10/56 = 18% O3-M122, 4/56 = 7.1% C1a1-M8, 2/56 = 3.6% O2a-K18, and 2/56 = 3.6% C2-M217.[69]
In a project approved by the Ethics Committee of Tokai University School of Medicine, Ochiai et al. (2016) have reported finding D-M174 (rs2032602 T>C) in 24/59 (40.7%), O-M268 (rs13447443 A>G) in 21/59 (35.6%), C-M130 (rs35284970 C>T) in 8/59 (13.6%), O-P198 (rs17269816 T>C) in 4/59 (6.8%), N-M231 (rs9341278 G>A) in 1/59 (1.7%), and O-P186(xM268, P198) (rs16981290 C>A, rs13447443 A, rs17269816 T) in 1/59 (1.7%) of a sample obtained through buccal swabs from Japanese male volunteers (n = 59) who had given informed consent to participate in the study.[51]
Maternal lineages
According to an analysis of the 1000 Genomes Project's sample of Japanese collected in the Tokyo metropolitan area, the mtDNA haplogroups found among modern Japanese include D (42/118 = 35.6%, including 39/118 = 33.1% D4 and 3/118 = 2.5% D5), B (16/118 = 13.6%, including 11/118 = 9.3% B4 and 5/118 = 4.2% B5), M7 (12/118 = 10.2%), G (12/118 = 10.2%), N9 (10/118 = 8.5%), F (9/118 = 7.6%), A (8/118 = 6.8%), Z (4/118 = 3.4%), M9 (3/118 = 2.5%), and M8 (2/118 = 1.7%).[70]
Single-nucleotide polymorphism
A 2011 SNP consortium study done by the Chinese Academy of Sciences and Max Planck Society consisting of 1719 DNA samples determined that Koreans and Japanese clustered near to each other, confirming the findings of an earlier study that Koreans and Japanese are related. However, the Japanese were found to be genetically closer to South Asian populations as evident by a genetic position that is significantly closer towards South Asian populations on the principal component analysis (PCA) chart. Some Japanese individuals are also genetically closer to Southeast Asian and Melanesian populations when compared to other East Asians such as Koreans and Han Chinese, indicating possible genetic interactions between Japanese and these populations.[71]
A 2008 study about genome-wide SNPs of East Asians by Chao Tian et al. reported that Japanese along with other East Asians such as Joseon Koreans and Han Chinese are genetically distinguishable from Southeast Asians and that the Japanese are related to Koreans, who in turn are more closely related to Han Chinese. However, the Japanese are relatively genetically distant from Han Chinese, compared to Koreans.[72] Another study (2017) shows a relative strong relation between all East and Southeast Asians.[73]
Immunoglobulin G
Hideo Matsumoto, professor emeritus at Osaka Medical College tested Gm types, genetic markers of immunoglobulin G, of Japanese populations for a 2009 study.[74] According to this study, the Gm ab3st gene is found at notably high frequencies across eastern Siberia, northern China, Korea, Mongolia, Japan, and Tibet.[74] The mean frequency of Gm ab3st for the mainstream Japanese population was found to be 26.0%, with a peak in the Yaeyama Islands (36.4% Yonaguni, 32.1% Ishigaki) among all populations in Japan and peaks in Akita (29.5%) and Shizunai (28.3%) among mainstream Japanese.[74] On mainland Asia, peak frequencies of Gm ab3st were found among Oroqen (44.0%) and Tungus (30.0%) in northeast China and among the north Baikal Buryats (30.7%); however, this gene is also frequent among Eskimos (25.4% Alaska, 24.7% Greenland, 20.5% Chaplin, Russia), Luoravetlans (Koryak 20.0%, Chukchi 15.3%), and Athabaskans (New Mexico Apache 19.7%, Alaska Athabascan 14.3%), and it is not uncommon even as far west as the south shore of the Caspian Sea (8.8% Gilani, 8.5% Mazanderani).[74] Minimum frequencies of Gm ab3st were found in Yakushima (22.0%) among all populations in Japan and in Tsu (23.3%) and Ōita (23.6%) among mainstream Japanese.[74] The data from small, isolated island populations, such as those of Yonaguni, Ishigaki, and Yakushima, were not used when calculating the mean for the mainstream Japanese population.[74] The study also considered Ainu and Korean populations and found Gm ab3st with a frequency of 25.2% among Ainu in Hidaka, Hokkaido and a mean frequency of 14.5% (range 13.1% Pusan, South Korea to 18.6% Yanji, China) among Koreans.[74]
Gm afb1b3, on the other hand, is a southern marker gene possibly originating in southern China on the background of the fb1b3 gene (the modal Gm type among Caucasoids) and found at very high frequencies across southern China, Southeast Asia, Taiwan, Sri Lanka, Bangladesh, Nepal, Assam, and the Pacific Islands.[74] Professor Matsumoto has remarked that the center of dispersal of the Gm afb1b3 gene may be in the Yunnan and Guangxi area of southern China; extremely high frequencies of this gene have been observed in samples of mostly Daic peoples from this region (95.2% Shui in Sandu, Guizhou, 94.2% Zhuang in Guangxi, 91.4% Bouyei in Duyun, Guizhou, 87.5% Miao in Guizhou, 84.0% Dai in Luxi, Yunnan) and from neighboring Laos (97.0% Laotian) and Thailand (89.9% Thai).[74] However, Gm afb1b3 is almost equally common among people in Malaysia (97.3% Kadazan on Borneo, 85.0% Malay), Indonesia (76.6% Sulawesi, 75.2% Java), the Philippines (83.6% Luzon Filipinos, 76.4% Luzon Negritos, 67.2% Mindanao Negritos), Karen people in Thailand (82.3%), Kacharis in Assam (80.9%), Cambodians (76.7%), Taiwanese aborigines (76.2%), Micronesians (88.7%), Melanesians (74.6%), and Polynesians (74.7% Cook Islands, 69.4% Hawaii).[74] The study found that the mean frequency of Gm afb1b3 was 10.6% (range 7.8% Shizunai to 13.0% Osaka) for the general Japanese population. Minimum frequencies (4.0% to 4.4%) of Gm afb1b3 were found among the native people in the Yaeyama and Miyako islands in the extreme south of Japan and among the Ainu (4.3%) in the extreme north of Japan. The author suggested that the somewhat elevated frequency of the Gm afb1b3 gene among the mainstream Japanese compared to the Sakishima islanders and the Ainu may have resulted from some admixture of the mainstream Japanese population at rates as low as 7–8% with southern Asian (from southern China or Southeast Asia as far west as Bangladesh and Nepal) populations having the Gm afb1b3 gene in high frequency.[74]
The other Gm types observed among Japanese are ag (45.8%) and axg (17.6%), which are not so useful for discerning human migrations and genetic relationships because they appear to be retained from a common ancestor of most modern humans and are found in similar proportions (with the frequency of ag being significantly greater than the frequency of axg) in many populations all over the world (aboriginal Australians and Americans, South Asians, Caucasoids, etc.).[74]
Genetic components compared with other Asian populations
A 2017 study conducted by Fumihiko Takeuchi, Tomohiro Katsuya, Ryosuke Kimura and Norihiro Kato compared three genetically distinct Japanese groups, Hondu (Honshu), Ryukyu and Ainu to 26 other Asian populations to analyze the shared ancestry and genetic differentiation between the Japanese people and other Asians.[75] The study revealed for the Japanese as a whole, some genetic components from all of the Central, East, Southeast and South Asian populations are prevalent in the Japanese population with the major components of ancestry profile coming from the Korean and Han Chinese clusters.[75] The major components of the Japanese Hondo cluster is similar to the Korean (87–94%), followed by Han Chinese 1 (0–8%) clusters.[75] The genetic components from the Southeast Asian (Thais, Vietnamese and Malays) and South Asian (Sinhalese and Tamils) clusters were larger for the Ryukyu cluster – Southeast Asian (4–6%) and South Asian (4–6%) – in comparison to the results found in the Hondo cluster – Southeast Asian (0–1%) and South Asian (1–2%).[75]
Predominantly Yayoi Origins of the Modern Japanese
A recent study (2018) shows that the Japanese are predominantly descendants of the Yayoi people and are closely related to other modern East Asians, especially Koreans and Han Chinese.[9][76] It is estimated that the majority of Japanese only has about 12% Jōmon ancestry or even less.[77]
Recent studies suggest that the Japanese people are predominantly descendants of the Yayoi people, and that the Yayoi largely displaced the local Jōmon.[9]
A genome research (Takahashi et al. 2019) shows that modern Japanese (Yamato) do not have much Jōmon ancestry at all. Nuclear genome analysis of Jōmon samples and modern Japanese samples show strong differences.[49]
A study by Gakihari et al. 2019 estimate the gene-flow from Jōmon people into modern Japanese people at about 3.3% or 9.8%, and that modern Japanese cluster closely with other East Asians but are clearly distinct from the Ainu people.[78] A study by Kanazawa-Kiriyama et al. (2019) estmates 9–13% ancestry from the Jomon in the modern Japanese.[79]
Ainu people
The Ainu people, an ethnic minority in Hokkaido, Sakhalin and the Kurils, were found to have formed from two distinct ancestry groups during the Jōmon period, one distinctive Paleolithic population from Central Asia and one ancient population from Northeast Asia (Okhotsk people, with both arriving at different times during the Jōmon period in Japan. According to Lee and Hasegawa from the Waseda University, the Ainu-speakers itself originated from the Northeast Asian/Okhotsk population, which established themselves in northern Hokkaido and expanded into large parts of Honshu and the Kurils and created the Incipient Jōmon culture, long before the arrival of contemporary Japanese people.[80][81][82] A study by Gakuhari et al. (2019) suggests about 79.3% ancestry from the Jomon in the modern Ainu.[83] A study by Kanazawa-Kiriyama et al. (2019) suggests about 66%.[84]
Genetic history of Koreans
Studies of polymorphisms in the human Y-chromosome have so far produced evidence to suggest that the Korean people have a long history as a distinct, mostly endogamous ethnic group, with successive waves of people moving to the peninsula and three major Y-chromosome haplogroups.[85] The reference population for Koreans used in Geno 2.0 Next Generation is 94% Eastern Asia and 5% Southeast Asia & Oceania.[86]
Several studies confirmed that Koreans are basically a Northeast Asian population, but that Korean populations have both Northeast and Southeast Asian genome.[87]
Paternal lineages
Jin Han-jun et al. (2003) said that the distribution of Y-chromosomal haplogroups shows that Koreans have a complex origin that results from genetic contributions from range expansions, most of which are from southern-to-northern China, and genetic contributions from the northern Asian settlement.[62]
Korean males display a high frequency of Haplogroup O-M176 (O1b2, formerly O2b), a subclade that probably has spread mainly from somewhere in the Korean Peninsula or its vicinity,[55][88] and Haplogroup O-M122 (O2, formerly O3), a common Y-DNA haplogroup among East and Southeast Asians in general.[89][12] Haplogroup O1b2-M176 has been found in approximately 30% (ranging from 20%[62][56] to 37%[59]) of sampled Korean males, while haplogroup O2-M122 has been found in approximately 40% of sampled Korean males.[56][90][66] Korean males also exhibit a moderate frequency (approximately 15%) of Haplogroup C-M217.
About 2% of Korean males belong to Haplogroup D-M174 (0/216 = 0.0% DE-YAP,[66] 3/300 = 1.0% DE-M145,[91] 1/68 = 1.5% DE-YAP(xE-SRY4064),[56] 8/506 = 1.6% D1b-M55,[55] 3/154 = 1.9% DE, 18/706 = 2.55% D-M174,[92] 5/164 = 3.0% D-M174,[64] 1/75 D1b*-P37.1(xD1b1-M116.1) + 2/75 D1b1a-M125(xD1b1a1-P42) = 3/75 = 4.0% D1b-P37.1,[59] 3/45 = 6.7% D-M174[93]). The D1b-M55 subclade has been found with maximal frequency in a small sample (n=16) of the Ainu people of Japan, and is generally frequent throughout the Japanese Archipelago.[94] Other haplogroups that have been found less commonly in samples of Korean males are Y-DNA haplogroup N-M231 (approx. 4%), haplogroup O-M119 (approx. 3%), haplogroup O-M268(xM176) (approx. 2%), haplogroup Q-M242 and Haplogroup R1 (approx. 2% total), J, Y*(xA, C, DE, J, K), L, C-RPS4Y(xM105, M38, M217), and C-M105.[55][56]
Korea Foundation Associate Professor of History, Eugene Y. Park, said that there is no correlation between a Korean person's Y-chromosome DNA haplogroup and their surname or ancestral seat.[95][96]
He Miao et al. (2009) created an artificial combination of equal parts of the Y-chromsomes of the HapMap samples of Han Chinese in Beijing and Japanese in Tokyo. The study said that this artificial combination resembled five populations which included Koreans in South Korea and Koreans in China.[97]
Maternal lineages
Studies of Korean mitochondrial DNA lineages have shown that there is a high frequency of Haplogroup D4, ranging from approximately 23% (11/48) among ethnic Koreans in Arun Banner, Inner Mongolia[98] to approximately 32% (33/103) among Koreans from South Korea.[63][99] Haplogroup D4 is the modal mtDNA haplogroup among northern East Asians (Japanese, Ryukyuans, Koreans, Manchus, Oroqens, Manchurian Evenks, Daurs, Mongols, northern Han Chinese, Tibetans) in general, with a peak frequency among Japanese and Ryukyuans in Japan. Haplogroup B, which occurs very frequently in many populations of Southeast Asia, Polynesia, and the Americas, is found in approximately 10% (5/48 ethnic Koreans from Arun Banner, Inner Mongolia) to 20% (21/103 Koreans from South Korea) of Koreans.[98][99] Haplogroup A has been detected in approximately 7% (7/103 Koreans from South Korea) to 15% (7/48 ethnic Koreans from Arun Banner, Inner Mongolia) of Koreans.[98][99][63] Haplogroup A is the most common mtDNA haplogroup among the Chukchi, Eskimo, Na-Dene, and many Amerind ethnic groups of North and Central America.
The other half of the Korean mtDNA pool consists of an assortment of various haplogroups, each found with relatively low frequency, such as G, N9, Y, F, D5, M7, M8, M9, M10, M11, R11, C, and Z.
Hwan Young Lee et al. (2006) studied a sample of 694 Koreans and found the following mtDNA distribution: 32.56% (226/694) D (including 188/694 = 27.09% D4, 37/694 = 5.33% D5, and 1/694 = 0.14% D6a), 14.84% (103/694) B (including 23/694 = 3.31% B4a, 22/694 = 3.17% B4b, 20/694 = 2.88% B5b, 12/694 = 1.73% B4c, 12/694 = 1.73% B4*, 10/694 = 1.44% B5a, 2/694 = 0.29% B4d, and 2/694 = 0.29% B4f), 9.65% (67/694) G (including 27/694 = 3.89% G2a, 18/694 = 2.59% G1a, 11/694 = 1.59% G3, 9/694 = 1.30% G*, 1/694 = 0.14% G2c, and 1/694 = 0.14% G4), 8.79% (61/694) A (including 23/694 = 3.31% A5a, 3/694 = 0.43% A5b, 3/694 = 0.43% A5c, 25/694 = 3.60% A4, and 7/694 = 1.01% A(xA4, A5)), 8.36% (58/694) F, 8.21% (57/694) N9 (including 18/694 = 2.59% N9a2a, 11/694 = 1.59% N9a2*, 11/694 = 1.59% N9a1, 9/694 = 1.30% Y1b, 4/694 = 0.58% N9a*, 2/694 = 0.29% N9b, and 2/694 = 0.29% Y2), 7.78% (54/694) M7 (including 25/694 = 3.60% M7b, 20/694 = 2.88% M7c, and 9/694 = 1.30% M7a), 4.76% (33/694) M8'CZ (including 17/694 = 2.45% C, 7/694 = 1.01% M8a, 7/694 = 1.01% Z, and 2/694 = 0.29% pre-Z), 1.87% (13/694) M9, 1.73% (12/694) M10, 0.72% (5/694) M11, 0.29% (2/694) R11, 0.14% (1/694) R9b, 0.14% (1/694) M12, and 0.14% (1/694) M*.[100]
Derenko et al. (2007) examined a sample of 103 Koreans from South Korea and found that they belonged to the following mtDNA haplogroups: 39.8% D (including 32.0% D4 and 7.8% D5), 20.4% B (including 12.6% B4 and 7.8% B5), 9.7% M7, 6.8% A (including 3.9% A5 and 2.9% A4(xA2, A8)), 6.8% G (including 2.9% G2a, 1.9% G1, and 1.9% G3), 4.9% F1, 4.9% M8a2, 2.9% N9a, 1.9% M9a, 1.0% C(xC1, C4, C5), and 1.0% Y.[99]
A study of the mtDNA of 708 Koreans sampled from six regions of South Korea (134 from Seoul-Gyeonggi, 118 from Jeolla, 117 from Chungcheong, 114 from Gangwon, 113 from Jeju, and 112 from Gyeongsang) found that they belonged to haplogroup D (35.5%, including 14.7% D4(xD4a, D4b), 7.8% D4a, 6.5% D5, 6.4% D4b, and 0.14% D(xD4, D5)), haplogroup B (14.8%, including 11.0% B4 and 3.8% B5), haplogroup A (8.3%), haplogroup M7 (7.6%), haplogroup F (7.1%), haplogroup M8'CZ (6.5%), haplogroup G (6.1%), haplogroup N9a (5.2%), haplogroup Y (3.8%), haplogroup M9 (2.7%), haplogroup M10 (1.6%), haplogroup M11 (0.42%), haplogroup N(xN9, Y, A, F, B4, B5) (0.28%), and haplogroup N9(xN9a) (0.14%).[101]
A study of 1094 individuals in the Korean Genome Project found that they belonged to haplogroup D (34.19%), haplogroup B (13.89%), haplogroup M(xC, D, G, Z) (13.8%), haplogroup A (8.32%), haplogroup G (8.23%), haplogroup F (7.86%), haplogroup N(xA, B, F, R, Y) (5.76%), haplogroup C (3.02%), haplogroup R(xB, F) (2.01%), haplogroup Y (1.74%), and haplogroup Z (1.19%).[102] The individuals sampled for the Korean Genome Project are mostly from the Ulsan metropolitan region.[102]
Autosomal DNA
Jin Han-jun et al. (1999) said that, based on genetic studies of classic genetic markers of protein and nuclear DNA, Koreans tend to be closely genetically related to Mongols among East Asians, which is supported by the following studies: Goedde et al. (1987); Saha & Tay (1992); Hong et al. (1993); and Nei & Roychoudhury (1993). The study said that the mtDNA 9‐bp deletion frequency in the intergenic COII/tRNALys region of Mongols (5.1%) is lower than that of Chinese (14.2%), Japanese (14.3%) and Koreans (15.5%). The study said that these 9‐bp deletion frequencies suggest that Koreans are closely related to Japanese and Chinese and that Koreans are not so closely related to Mongols. The study said that the homogeneity in the 9-bp deletion frequencies among Chinese (14.2%), Japanese (14.3%) and Koreans (15.5%), only spanning from a low of 14.2% for Chinese to a high of 15.5% for Koreans, indicates that very few mtDNA are differentiated in these three populations. The study said that the 9‐bp deletion frequencies for Vietnamese (23.2%) and Indonesians (25.0%), which are the two populations constituting Mongoloid Southeast Asians in the study, are relatively high frequencies when compared to the 9-bp deletion frequencies for Mongols(5.1%), Chinese (14.2%), Japanese (14.3%) and Koreans (15.5%), which are the four populations constituting Northeast Asians in the study. The study said that these 9-bp deletion frequencies are consistent with earlier surveys which showed that 9-bp deletion frequencies increase going from Japan to mainland Asia to the Malay Peninsula, which is supported by the following studies: Horai et al. (1987); Hertzberg et al. (1989); Stoneking & Wilson (1989); Horai (1991); Ballinger et al. (1992); Hanihara et al. (1992); and Chen et al. (1995). The study said that Cavalli-Sforza's chord genetic distance (4D), from Cavalli-Sforza & Bodmer (1971), which is based on the allele frequencies of the intergenic COII/tRNALys region, showed that Koreans are more genetically related to Japanese than Koreans are genetically related to the other East Asian populations which were surveyed. The Cavalli-Sforza's chord genetic distance (4D) between Koreans and other East Asian populations in the study, from least to greatest, are as follows: Korean to Japanese (0.0019), Korean to Chinese (0.0141), Korean to Vietnamese (0.0265), Korean to Indonesian (0.0316) and Korean to Mongols (0.0403). The study said that the close genetic affinity between present-day Koreans and Japanese is expected due to the Yayoi migration from China and the Korean Peninsula to Japan which began about 2,300 years ago, a migration which is supported by the following studies: Chard (1974); Hanihara (1991); Hammer & Horai (1995); Horai et al. (1996); Omoto & Saitou (1997). The study said that Horai et al. (1996) detected mtDNA D-loop variation which supports the idea that a large amount of maternal lineages came into Japan from immigrants from the Korean Peninsula after the Yayoi period.[103]
Wook et al. (2000) said that Chu et al. (1998) found that phylogeny which was based on 30 microsatellites indicated that Korean people were closely related to Chinese people from Manchuria and Yunnan, but Kim Wook et al. (2000) found that the high incidence of the DXYS156Y-null variant in northeast Chinese implied that it is possible to exclude these northeastern Chinese populations from being sources which are significant in Korean people. The phylogenetic analysis done by Wook et al. (2000) indicated that Japanese people are genetically closer to Korean people than Japanese people are genetically related to any of the following peoples: Mongolians, Chinese, Vietnamese, Indonesians, Filipinos and Thais. The study said that mainland Japanese having Koreans as their closest genetic population is consistent with the following previous studies: Hammer and Horai (1995); Horai et al. (1996); and Kim et al. (1998). The study found that Koreans are more genetically homogenous than the Japanese, and the study said that this might be due to different sizes of the founding populations and range expansions. The study said that the moderate mean Y-chromosome haplotype diversity value for Koreans might be the result of migrations from East Asia that had a homogenizing influence. The study said that it is more probable that Koreans descend from dual infusions of Y-chromosomes from two different waves of East Asians rather than a single East Asian population due to the dual patterns of the Y-chromosome haplotype distribution found in Koreans.[104]
Kim Jong-jin et al. (2005) did a study about the genetic relationships among East Asians based on allele frequencies, particularly focusing on how close Chinese, Japanese and Koreans are genetically related to each other. Most Koreans were hard to distinguish from Japanese, and the study was not able to clearly distinguish Koreans and Japanese. Koreans and Japanese clustered together in the principal component analysis and the best least-squares tree. The study said that "[c]ommon ancestry and/or extensive gene flow" historically between Koreans and Japanese appears to be "likely" and results in a lot of difficulty finding population-specific alleles that could assist in differentiating Koreans and Japanese.[105]
Jung Jongsun et al. (2010) used the following Korean samples for a study: Southeast Korean (sample regions: Gyeongju, Goryeong and Ulsan), Middle West Korean (sample regions: Jecheon, Yeoncheon, Cheonan and Pyeongchang) and Southwest Korean (sample regions: Gimje, Naju and Jeju). Due to political reasons, the study said that it did not use North Korean samples, but the study said that the "historical migration event of Baekje from Goguryeo Empire (BC 37–AD 668) in Northern Korea imply that Northern lineages remain in South Korea." The study said that the "Northern people of the Goguryeo Empire" are closely related to Mongolians, and the study said that this group of people ruled most of Southwest Korea. The study said that "some of the royal families and their subjects in the Goguryeo Empire moved to this region and formed the Baekje Empire in BC 18–22." Southwest Koreans are closer to Mongolians in the study's genome map than the other two Korean regions in the study are close to Mongolians. Southwest Koreans also display genetic connections with the HapMap sample of Japanese in Tokyo, and, in the neighbor joining tree, the nodes for Southwest Korea are close to Japan. In the study's Korea-China-Japan genome map, some Southwest Korean samples overlap with samples from Japan. The study said that the fairly close relationship, in both the study's genetic structure analysis and genome map, of the Jeju Southwest Korean sample and the HapMap sample of Japanese in Tokyo, Japan, has made the evolutionary relationship of Chinese, Japanese and Koreans become clearer. Southeast Koreans display some genetic similarity with people of Kobe, Japan, which indicates that there might have been links between these regions. The study said that it is possible that outliers in the Gyeongju sample, one of the sampled Southeast Korean regions, and outliers in the Kobe, Japan, sample both have Siberian lineage due to Southeast Koreans having connections with Siberian lineages with respect to grave patterns and culture. The overall result for the study's Korea-Japan-China genome map indicates that some signals for Siberia remain in Southeast Korea. In contrast to the Gyeongju sample, the Goryeong and Ulsan samples, which are both Southeast Korean samples, displayed average signals for the Korean Peninsula. The study said that Middle West Korea was a melting pot in the Korean Peninsula with people traveling from North to South, South to North, and people traveling from East China, including from the Shandong Peninsula. Western Chinese, which included those in the Shandong Peninsula, travelled across the Yellow Sea, and these Western Chinese lived and traded in both China and Korea. In the study's genome map, Middle West Koreans are close to the HapMap sample of Han Chinese in Beijing and, in the neighbor joining tree, the nodes for Middle West Korea are close to China. The overall result for the study's Korea-Japan-China genome map indicates that Middle West Korea displays an average signal for South Korea. Chinese people are located between Korean and Vietnamese people in the study's genome map.[106][107]
Kim Young-jin and Jin Han-jun (2013) said that principal component analysis had Korean HapMap samples clustering with neighboring East Asian populations which were geographically nearby them such as the Chinese and Japanese. The study said that Koreans are genetically closely related to Japanese in comparison to Koreans' genetic relatedness to other East Asians which included the following East and Southeast Asian peoples: Tujia, Miao, Daur, She, Mongols, Naxi, Cambodians, Oroqen, Yakuts, Yi, Southern Han Chinese, Northern Han Chinese, Hezhen, Xibo, Lahu, Dai and Tu. The study said that the close genetic relatedness of Koreans to Japanese has been reported in the following previous studies: Kivisild et al. (2002); Jin et al. (2003); Jin et al. (2009); and Underhill and Kivisild (2007). The study said that Jung et al. (2010) said that there is a genetic substructure in Koreans, but the study said that it found Korean HapMap individuals to be highly genetically similar. The study said that Jin et al. (2009) found that Koreans from different populations are not different in a significant way which indicates that Koreans are genetically homogenous. The study said that the affinity of Koreans is predominately Southeast Asian with an estimated admixture of 79% Southeast Asian and 21% Northeast Asian for Koreans, but the study said that this does not mean that Koreans are heterogenous, because all of the Koreans which were analyzed uniformly displayed a dual pattern of Northeast Asian and Southeast Asian origins. The study said that Koreans and Japanese displayed no observable difference between each other in their proportion of Southeast Asian and Northeast Asian admixture. The study said the 79% Southeast Asian and 21% Northeast Asian admixture estimate for Koreans is consistent with the interpretation of Jin et al. (2009) that Koreans descend from a Northeast Asian population which was subsequently followed by a male-centric migration from the southern region of Asia which changed both the autosomal composition and Y-chromosomes in the Korean population.
Veronika Siska et al. (2017) said that the Ulchi people are genetically closest in the study's panel to the human remains from the Devil's Gate Cave which are dated to about 7,700 years ago. Modern Korean and Japanese, the Oroqen people and the Hezhen people display a high affinity to the human remains from Devil's Gate Cave. Considering the geographic distance of Amerindians from Devil's Gate Cave, Amerindians are unusually genetically close to the human remains from Devil's Gate Cave. Korean genomes display similar traits to Japanese genomes on genome-wide SNP data. In an admixture analysis, when the genes of Devil's Gate is made into a unique genetic component, this new Devil's Gate genetic component is highest in peoples of the Amur Basin, including Ulchi, and makes up about more than 50% of Koreans and Japanese. It also has a sporadic distribution among other East Asians, Central Asians and Southeast Asians.[108]
Immunoglobulin G
Hideo Matsumoto, professor emeritus at Osaka Medical College, tested Gm types, genetic markers of immunoglobulin G, of Korean populations for a 2009 study. The Korean populations were populations in Jeju Island, Busan, Gwangju, Kongsan, Jeonju, Wonju, the Kannung of South Korea and a Korean population in Yanji. Matsumoto said that the Gm ab3st gene is a marker for northern Mongoloid possibly originating in Siberia and found at high frequencies across northeast Asia and Tibet. Matsumoto said that the average frequency of Gm ab3st for Koreans was 14.5% which was intermediate between an average frequency of 26% for general Japanese and a frequency of 11.7% which was for a Han Chinese population in Beijing. Matsumoto said that Gm afb1b3 is a southern marker gene possibly originating in southern China and found at high frequencies across Southeast Asia, southern China, Taiwan, Sri Lanka, Bangladesh, Nepal, Assam and parts of the Pacific. However, given the result that the Okinawans being genetically most northern among the Japanese with the highest frequency of the Gm ab3st gene which is assigned to be northern, the term northern and southern used in his study is controversial. Matsumoto said that the average frequency of Gm afb1b3 for Koreans was 14.7% which was intermediate between a frequency of 10.6% for general Japanese and a frequency of 24.1% for Beijing Han Chinese. Matsumoto said that Koreans displayed the northern Mongoloid pattern, but Matsumoto said that Koreans displayed a higher frequency of the southern marker gene, Gm afb1b3, than the Japanese. Matsumoto said that "Japanese and Korean populations were originally identical or extremely close to each other", and Matsumoto said, "It seemed to be during the formation of the contemporary Korean population that such a Gm pattern intermediate between Japanese and the northern Han in China emerged." Matsumoto said that the different Gm pattern between Japanese and Koreans most likely came about from frequent inflows of Chinese and/or northern populations into the Korean Peninsula.[109]
Genetic history of Mongolians
The Mongols are an ethnic group in northern China, Mongolia, parts of Siberia and Western Asia. They are believed to be the descendants of the Xianbei and the proto-Mongols. The former term includes the Mongols proper (also known as the Khalkha Mongols), Buryats, Oirats, the Kalmyk people and the Southern Mongols. The latter comprises the Abaga Mongols, Abaganar, Aohans, Baarins, Gorlos Mongols, Jalaids, Jaruud, Khishigten, Khuuchid, Muumyangan and Onnigud. The Daur people are descendants of the para-Mongolic Khitan people.[110] Mongolians are also related to the Manchurians.
Paternal lineages
The majority of Mongolians belong to the y-DNA Haplogroup C-M217. Haplogroup C-M217 among the Mongols is characterized by very deep total diversity that dates back to the very origin of haplogroup C-M217 (TMRCA 33,900 [95% CI 31,300 <-> 36,500] ybp[111]) and very shallow diversity in each of the frequently observed subclades: C-M504 (TMRCA 2,900 [95% CI 2,200 <-> 3,700] ybp), C-M86 (TMRCA 3,700 [95% CI 3,000 <-> 4,500] ybp[111]), C-M407 (TMRCA 4,400 [95% CI 3,500 <-> 5,200] ybp[111]), and C-F1756 (TMRCA 5,000 [95% CI 4,200 <-> 5,800] ybp[111]). Of these four subclades, C-M407 is phylogenetically extremely divergent from the others, and is more closely related to subclades of C-M217 that are found among present-day Chinese, Koreans, Japanese, and other East and Southeast Asians; however, among Mongols, C-M407 is found most frequently toward the north (among Barghuts[112] and Buryats[113] as well as the neighboring Khamnigans and Soyots) and toward the west (among Dorbet Kalmyks[114][115]).
Haplogroup O-M175 and Haplogroup N-M231 are found at medium rates among present-day Mongols. The subclades of Haplogroup O-M175 that have been observed among Mongols tend to be similar to those found among Han Chinese, whereas the subclades of Haplogroup N-M231 that have been observed among Mongols tend to be similar to those found among Nenets, Nganasans, Khakasses, and Tuvans (N-B478) on the one hand or those found among Chukchi, Koryaks, and Asian Eskimos on the other (N-B197).[116] However, N-M2118, a subclade which is most often noted for its extremely high frequency among present-day populations of Yakutia, has been observed in 21.4% (6/28) of a sample of Kalmyk Khoshut,[115] and N-B525 is also widely observed among Mongols with low frequency. In addition, some members of a wide variety of other Y-DNA haplogroups have been found among present-day Mongols, including Haplogroup Q-M242, Haplogroup R-M207 (Haplogroup R1b-M478, Haplogroup R1b-M269, Haplogroup R1a-M17, Haplogroup R2a-M124), Haplogroup D-M174, Haplogroup J2a-M410, Haplogroup J1-Page8, Haplogroup G1-M285, and Haplogroup I2a2-M436.[117]
Maternal lineages
The maternal haplogroups are diverse but similar to other northern Asian populations. The most common maternal haplogroups in Mongolians are haplogroup D4, Haplogroup A, and Haplogroup B.[118]
West Eurasian mtDNA haplogroups Haplogroup HV, Haplogroup U, Haplogroup K, Haplogroup I, Haplogroup J, represents 14% in western Xingjang Mongolian, 10% in Mongolia, 8.4% in central Inner Mongolian samples, 2% in eastern Xin Barage Zuoqi County samples.[119]
Genetic history of Tibetans
Modern Tibetan populations are genetically most similar to other modern East Asian populations.[120] They also show more genetic affinity for modern Central Asian than modern Siberian populations.[120]
A 2016 study found that the Tibetan gene pool diverged from that of Han Chinese around 15,000 years ago, which can be largely attributed to post-LGM (Last Glacial Maximum) arrivals. Analysis of around 200 contemporary populations showed that Tibetans share ancestry with populations from East Asia (~82%), Central Asia and Siberia (~11%), South Asia (~6%), and western Eurasia and Oceania (~1%). These results support that Tibetans arose from a mixture of multiple ancestral gene pools but that their origins are much more complicated and ancient than previously suspected.[120]
Relationship to other populations
A study in 2010 suggested that the majority of the Tibetan gene pool may have diverged from the Zang around 15,000 years ago.[121] However, there are possibilities of much earlier human inhabitation of Tibet,[122][123] and these early residents may have contributed to the modern Tibetan gene pool.[124]
The date of divergence between Tibetans and Sherpas was estimated to have taken place around 11,000 to 7,000 years ago.[120]
Relationship to archaic hominins
After modern Oceanic populations, modern Tibetan populations show the highest rate of allele sharing with archaic hominins at over 6%.[120] Modern Tibetans show genetic affinities to three archaic populations: Denisovans, Neanderthals, and an unidentified archaic population.[120]
In comparison to modern Han populations, modern Tibetans show greater genetic affinity to Denisovans; however, both the Han and Tibetans have similar ratios of genetic affinity to general Neanderthal populations.[120]
Modern Tibetans were identified as the modern population that has the most alleles in common with Ust'-Ishim man.[120]
Paternal lineage
The distribution of Haplogroup D-M174 (subclade Haplogroup D-Z27276) is found among nearly all the populations of Central Asia and Northeast Asia south of the Russian border, although generally at a low frequency of 2% or less. A dramatic spike in the frequency of D-M174 occurs as one approaches the Tibetan Plateau. D-M174 is also found at high frequencies among Japanese people, but it fades into low frequencies in Korea and China proper between Japan and Tibet. The claim that the Navajo people and Tibetans are related, while discussed among linguists since Edward Sapir, has not found support in genetic studies. Some light has been shed on their origins, however, by one genetic study in which it was indicated that Tibetan Y-chromosomes had multiple origins, one from Central Asia and the other from East Asia.[125]
Genetic history of Turks
The Turkic peoples are a collection of ethno-linguistic groups of Central-, Eastern-, Northern- and Western-Asia as well as parts of Europe and North Africa. They speak related languages belonging to the Turkic language family.
Proposals for the homeland of the Turkic peoples and their language are far-ranging, from the Transcaspian steppe to Northeastern Asia (Manchuria).[126]
According to Yunusbayev, genetic evidence points to an origin in the region near South Siberia and Mongolia as the "Inner Asian Homeland" of the Turkic ethnicity.[127]
Authors Joo-Yup Lee and Shuntu Kuang analyzed 10 years of genetic research on Turkic people and compiled scholarly information about Turkic origins, and said that the early and medieval Turks were a heterogeneous group and that the Turkification of Eurasia was a result of language diffusion, not a migration of homogeneous population.[128]
Paternal lineages
Common Y-DNA haplogroups in Turkic peoples are Haplogroup N-M231 (found with especially high frequency among Turkic peoples living in present-day Russia), Haplogroup C-M217 (especially in Central Asia and, in particular, Kazakhstan), Haplogroup Q-M242 (especially in Southern Siberia and among Turkmens and the Qangly tribe of Kazakhs), and Haplogroup O-M175 (especially among Turkic peoples living in present-day China and the Naiman tribe of Kazakhs). Some groups also have Haplogroup R1b (notably frequent among the Teleuts and Kumandins of Southern Siberia, the Bashkirs of the Southern Ural region of Russia, and the Qypshaq tribe of Kazakhs), Haplogroup R1a (notably frequent among the Kyrgyz, Altaians, and several other Turkic peoples living in present-day Russia), Haplogroup J-M172 (especially frequent among Uyghurs, Uzbeks, Azerbaijanis, and Turkish people), and Haplogroup D-M174 (especially among Yugurs, but also observed regularly with low frequency among Uyghurs, Southern Altaians, Nogais, Kazakhs, and Uzbeks).[129][130]
Anatolian/European Turks
The modern Turkic groups in Anatolia (Turkey) and Europe have less relation to East-Asian groups than their Central-Asian relatives. Various studies estimate about 15-30% East-Asian lineages in Anatolian/European Turks with the average at 21.7%.[131] A study in 2015 found that "Previous genetic studies have generally used Turks as representatives of ancient populations from Turkey. Our results show that Turks are genetically shifted towards modern Central Asians, a pattern consistent with a history of mixture with populations from this region". The authors found "7.9% (±0.4) East Asian ancestry in Turks from admixture occurring 800 (±170) years ago."[132] A 2019 study found that Turkish people cluster with Southern and Mediterranean Europe populations along with groups in the northern part of Southwest Asia (such as the populations from Caucasus, Northern Iraq, and Iranians).[133] Another study found the Circassians are closest to the Turkish population among sampled European (French, Italian, Sardinian), Middle Eastern (Druze, Palestinian), and Central (Kyrgyz, Hazara, Uygur), South (Pakistani), and East Asian (Mongolian, Han) populations.[134] Another 2019 study found that Turkish people have the lowest Fst distances with Caucasus population group and Iranian-Syrian group, compared to East-Central European, European (including Northern and Eastern European), Sardinian, Roma, and Turkmen groups or populations. Caucasus group in the study included samples from "Abkhazians, Adygey, Armenians, Balkars, Chechens, Georgians, Kumyks, Kurds, Lezgins, Nogays, and North Ossetia."[135]
A study involving mitochondrial analysis of a Byzantine-era population, whose samples were gathered from excavations in the archaeological site of Sagalassos, found that Sagalassos samples were closest to modern samples from "Turkey, Crimea, Iran and Italy (Campania and Puglia), Cyprus and the Balkans (Bulgaria, Croatia and Greece)."[136] Modern-day samples from the nearby town of Ağlasun showed that lineages of East Eurasian descent assigned to macro-haplogroup M were found in the modern samples from Ağlasun. This haplogroup is significantly more frequent in Ağlasun (15%) than in Byzantine Sagalassos, but the study concluded that there is "no genetic discontinuity across two millennia in the region."[137] Another study concluded that the true Central Asian contributions to Anatolia was 13% for males and 22% for females (with wide ranges of confidence intervals), and the language replacement in Turkey and Azerbaijan might not have been in accordance with the elite dominance model.[138]
Be that as it may, the most common male haplogroup in Anatolia is J2=24% - J2 (M172)[139] J-M172 (or J2) may reflect the spread of Anatolian farmers.[140] J2-M172 is "mainly confined to the Mediterranean coastal areas, southeastern Europe and Anatolia", as well as West Asia and Central Asia.[141]
Relationship to other Asian groups
Central Asians
The genetic evidence suggests that the Turkification of Central Asia was carried out by East Asian dominant minorities migrating out of Mongolia.[142] According to a recent study, the Turkic Central Asian populations, such as Kyrgyz, Kazakhs, Uzbeks, and Turkmens share more of their gene pool with various East Asian and Siberian populations than with West Asian or European populations. The study further suggests that both migration and linguistic assimilation helped to spread the Turkic languages in Eurasia.[143]
South Asians
According to a genetic research (2015) including linguistic analyses, suggests an East Asian origin for proto-Austroasiatic groups, which first migrated to Southeast Asia and later into India.[144] According to Ness, there are three broad theories on the origins of the Austroasiatic speakers, namely northeastern India, central or southern China, or southeast Asia.[145] Multiple researches indicate that the Austroasiatic populations in India are derived from (mostly male dominated) migrations from southeast Asia during the Holocene.[146][147][148][144][149][150] According to Van Driem (2007), "...the mitochondrial picture indicates that the Munda maternal lineage derives from the earliest human settlers on the Subcontinent, whilst the predominant Y chromosome haplogroup argues for a Southeast Asian paternal homeland for Austroasiatic language communities in India."[151]
According to Chaubey et al. (2011), "Austroasiatic speakers in India today are derived from dispersal from Southeast Asia, followed by extensive sex-specific admixture with local Indian populations."[147] According to Zhang et al. (2015), Austroasiatic (male) migrations from southeast Asia into India took place after the lates Glacial maximum, circa 10,000 years ago.[144] According to Arunkumar et al. (2015), Y-chromosomal haplogroup O2a1-M95, which is typical for Austrosiatic speaking peoples, clearly decreases from Laos to east India, with "a serial decrease in expansion time from east to west," namely "5.7 ± 0.3 Kya in Laos, 5.2 ± 0.6 in Northeast India, and 4.3 ± 0.2 in East India." This suggests "a late Neolithic east to west spread of the lineage O2a1-M95 from Laos."[149][152] According to Riccio et al. (2011), the Munda people are likely descended from Austroasiatic migrants from southeast Asia.[148][153] According to Ness, the Khasi probably migrated into India in the first millennium BCE.[145]
Southeast Asians
A 2020 genetic study about Southeast Asian populations in 2020 (Liu et al. 2020), found that mostly all Southeast Asians are closely related to East Asians and have mostly "East Asian-related" ancestry. Austronesian and Austroasiatic speaking populations of Southeast Asia were found to have mostly East Asian-related ancestry (89% to 96%) and minor Onge-related ancestry (1% to 11%). Additionally they found evidence for ancient gene flow from East Asian-related groups into the Andamanese people. Andamanese (Onge) were found to have about 30% East Asian-related ancestry next to their original Negrito ancestry. Taiwanese indigenous peoples had on average 99% East Asian-related ancestry. Kra-Dai speaking populations had, similar to the Taiwanese indigenous peoples, nearly exclusively East Asian-related ancestry.[154]
Notes
References
- "Xiongnu People". britannica.com. Encyclopædia Britannica. Retrieved 25 July 2015.
- di Cosmo 2004: 186
- Keyser-Tracqui C, Crubézy E, Ludes B (August 2003). "Nuclear and mitochondrial DNA analysis of a 2,000-year-old necropolis in the Egyin Gol Valley of Mongolia". American Journal of Human Genetics. 73 (2): 247–60. doi:10.1086/377005. PMC 1180365. PMID 12858290.
- Lihongjie, Y-Chromosome Genetic Diversity of the Ancient North Chinese populations, Jilin University-China (2012)
- Anthropology of Archaeological Populations from East- and Central-Asia* Archived 2013-07-29 at the Wayback Machine
- Changchun Y, Li X, Xiaolei Z, Hui Z, Hong Z (November 2006). "Genetic analysis on Tuoba Xianbei remains excavated from Qilang Mountain Cemetery in Qahar Right Wing Middle Banner of Inner Mongolia". FEBS Letters. 580 (26): 6242–6. doi:10.1016/j.febslet.2006.10.030. PMID 17070809. S2CID 19492267.
- Zhou H (March 2014). "Genetic analyses of Xianbei populations about 1,500–1,800 years old". Human Genetics. 50 (3): 308–314. doi:10.1134/S1022795414030119. S2CID 18809679.
- Changchun Y, Li X, Xiaolei Z, Hui Z, Hong Z (November 2006). "Genetic analysis on Tuoba Xianbei remains excavated from Qilang Mountain Cemetery in Qahar Right Wing Middle Banner of Inner Mongolia". FEBS Letters. 580 (26): 6242–6. doi:10.1016/j.febslet.2006.10.030. PMID 17070809. S2CID 19492267.
- Wang Y, Lu D, Chung YJ, Xu S (2018-04-06). "Genetic structure, divergence and admixture of Han Chinese, Japanese and Korean populations". Hereditas. 155 (1): 19. doi:10.1186/s41065-018-0057-5. PMC 5889524. PMID 29636655. [tp://blogs.biomedcentral.com/on-biology/2018/04/10/common-ancestor-of-han-chinese-japanese-and-koreans-dated-to-3000-3600-years-ago/ Lay summary] – blogs.biomedcentral.com.
- Zhao YB, Zhang Y, Zhang QC, Li HJ, Cui YQ, Xu Z, Jin L, Zhou H, Zhu H (2015). "Ancient DNA reveals that the genetic structure of the northern Han Chinese was shaped prior to 3,000 years ago". PLOS ONE. 10 (5): e0125676. Bibcode:2015PLoSO..1025676Z. doi:10.1371/journal.pone.0125676. PMC 4418768. PMID 25938511.
- Li J, Zeng W, Zhang Y, Ko AM, Li C, Zhu H, Fu Q, Zhou H (December 2017). "Ancient DNA reveals genetic connections between early Di-Qiang and Han Chinese". BMC Evolutionary Biology. 17 (1): 239. doi:10.1186/s12862-017-1082-0. PMC 5716020. PMID 29202706.
- Wen B, Li H, Lu D, Song X, Zhang F, He Y, Li F, Gao Y, Mao X, Zhang L, Qian J, Tan J, Jin J, Huang W, Deka R, Su B, Chakraborty R, Jin L (September 2004). "Genetic evidence supports demic diffusion of Han culture". Nature. 431 (7006): 302–5. Bibcode:2004Natur.431..302W. doi:10.1038/nature02878. PMID 15372031. S2CID 4301581.
- Du R, Xiao C, Cavalli-Sforza LL (December 1997). "Genetic distances between Chinese populations calculated on gene frequencies of 38 loci". Science in China Series C: Life Sciences. 40 (6): 613–21. doi:10.1007/BF02882691. PMID 18726285. S2CID 1924085.
- "World ancestry". admixturemap.paintmychromosomes.com. Retrieved 2016-02-09.
- Chen J, Zheng H, Bei JX, Sun L, Jia WH, Li T, Zhang F, Seielstad M, Zeng YX, et al. (December 2009). "Genetic structure of the Han Chinese population revealed by genome-wide SNP variation". American Journal of Human Genetics. 85 (6): 775–85. doi:10.1016/j.ajhg.2009.10.016. PMC 2790583. PMID 19944401.
- Gan RJ, Pan SL, Mustavich LF, Qin ZD, Cai XY, Qian J, Liu CW, Peng JH, Li SL, Xu JS, Jin L, Li H (2008). "Pinghua population as an exception of Han Chinese's coherent genetic structure". Journal of Human Genetics. 53 (4): 303–13. doi:10.1007/s10038-008-0250-x. PMID 18270655.
- He JD, Peng MS, Quang HH, Dang KP, Trieu AV, Wu SF, et al. (May 7, 2012). Kayser M (ed.). "Patrilineal perspective on the Austronesian diffusion in Mainland Southeast Asia". PLOS ONE. 7 (5): e36437. Bibcode:2012PLoSO...736437H. doi:10.1371/journal.pone.0036437. PMC 3346718. PMID 22586471.
- Pischedda S, Barral-Arca R, Gómez-Carballa A, Pardo-Seco J, Catelli ML, Álvarez-Iglesias V, et al. (October 2017). "Phylogeographic and genome-wide investigations of Vietnam ethnic groups reveal signatures of complex historical demographic movements". Scientific Reports. 7 (1): 12630. Bibcode:2017NatSR...712630P. doi:10.1038/s41598-017-12813-6. PMC 5626762. PMID 28974757.
- Liu D, Duong NT, Ton ND, Van Phong N, Pakendorf B, Van Hai N, Stoneking M (April 2020). "Extensive ethnolinguistic diversity in Vietnam reflects multiple sources of genetic diversity". Molecular Biology and Evolution. 37 (9): 2503–2519. doi:10.1093/molbev/msaa099. PMC 7475039. PMID 32344428.
- Hurles ME, Sykes BC, Jobling MA, Forster P (May 2005). "The dual origin of the Malagasy in Island Southeast Asia and East Africa: evidence from maternal and paternal lineages". American Journal of Human Genetics. 76 (5): 894–901. doi:10.1086/430051. PMC 1199379. PMID 15793703.
- Lu C, Zhang J, Li Y, Xia Y, Zhang F, Wu B, Wu W, Ji G, Gu A, Wang S, Jin L, Wang X (March 2009). "The b2/b3 subdeletion shows higher risk of spermatogenic failure and higher frequency of complete AZFc deletion than the gr/gr subdeletion in a Chinese population". Human Molecular Genetics. 18 (6): 1122–30. doi:10.1093/hmg/ddn427. PMID 19088127.
- Xue F, Wang Y, Xu S, Zhang F, Wen B, Wu X, Lu M, Deka R, Qian J, et al. (June 2008). "A spatial analysis of genetic structure of human populations in China reveals distinct difference between maternal and paternal lineages". European Journal of Human Genetics. 16 (6): 705–17. doi:10.1038/sj.ejhg.5201998. PMID 18212820.
- Wen B, Li H, Lu D, Song X, Zhang F, He Y, Li F, Gao Y, Mao X, et al. (September 2004). "Genetic evidence supports demic diffusion of Han culture". Nature. 431 (7006): 302–5. Bibcode:2004Natur.431..302W. doi:10.1038/nature02878. PMID 15372031. S2CID 4301581.
- Li H, Wen B, Chen SJ, Su B, Pramoonjago P, Liu Y, Pan S, Qin Z, Liu W, Cheng X, Yang N, Li X, Tran D, Lu D, Hsu MT, Deka R, Marzuki S, Tan CC, Jin L (May 2008). "Paternal genetic affinity between Western Austronesians and Daic populations". BMC Evolutionary Biology. 8 (1): 146. doi:10.1186/1471-2148-8-146. PMC 2408594. PMID 18482451.
- Karafet TM, Hallmark B, Cox MP, Sudoyo H, Downey S, Lansing JS, Hammer MF (August 2010). "Major east-west division underlies Y chromosome stratification across Indonesia". Molecular Biology and Evolution. 27 (8): 1833–44. doi:10.1093/molbev/msq063. PMID 20207712.
- Edén CS, Hagberg L, Hanson LA, Korhonen T, Leffler H, Olling S (2008). "Adhesion of Escherichia coli in urinary tract infection". Ciba Foundation Symposium. Novartis Foundation Symposia. 80: 161–87. doi:10.1002/9780470720639.ch11. ISBN 9780470720639. PMID 6114819.
- Wang X. "Han Chinese dialect area by the distribution of the Y chromosome". Blog.ifeng.com. Wang Xiadong. Archived from the original on 14 July 2014. Retrieved 10 June 2014.
- Yan S, Wang CC, Li H, Li SL, Jin L (September 2011). "An updated tree of Y-chromosome Haplogroup O and revised phylogenetic positions of mutations P164 and PK4". European Journal of Human Genetics. 19 (9): 1013–5. doi:10.1038/ejhg.2011.64. PMC 3179364. PMID 21505448.
- Yao YG, Kong QP, Bandelt HJ, Kivisild T, Zhang YP (March 2002). "Phylogeographic differentiation of mitochondrial DNA in Han Chinese". American Journal of Human Genetics. 70 (3): 635–51. doi:10.1086/338999. PMC 384943. PMID 11836649.
- Kivisild T, Tolk HV, Parik J, Wang Y, Papiha SS, Bandelt HJ, Villems R (October 2002). "The emerging limbs and twigs of the East Asian mtDNA tree". Molecular Biology and Evolution. 19 (10): 1737–51. doi:10.1093/oxfordjournals.molbev.a003996. PMID 12270900.
- Yao YG, Kong QP, Man XY, Bandelt HJ, Zhang YP (February 2003). "Reconstructing the evolutionary history of China: a caveat about inferences drawn from ancient DNA". Molecular Biology and Evolution. 20 (2): 214–9. doi:10.1093/molbev/msg026. PMID 12598688.
- Kong QP, Sun C, Wang HW, Zhao M, Wang WZ, Zhong L, Hao XD, Pan H, Wang SY, Cheng YT, Zhu CL, Wu SF, Liu LN, Jin JQ, Yao YG, Zhang YP (January 2011). "Large-scale mtDNA screening reveals a surprising matrilineal complexity in east Asia and its implications to the peopling of the region". Molecular Biology and Evolution. 28 (1): 513–22. doi:10.1093/molbev/msq219. PMID 20713468.
- Wei LH, Yan S, Yu G, Huang YZ, Yao DL, Li SL, et al. (March 2017). "Genetic trail for the early migrations of Aisin Gioro, the imperial house of the Qing dynasty". Journal of Human Genetics. 62 (3): 407–411. doi:10.1038/jhg.2016.142. PMID 27853133. S2CID 7685248.
- Yan S, Tachibana H, Wei LH, Yu G, Wen SQ, Wang CC (June 2015). "Y chromosome of Aisin Gioro, the imperial house of the Qing dynasty". Journal of Human Genetics. 60 (6): 295–8. arXiv:1412.6274. doi:10.1038/jhg.2015.28. PMID 25833470. S2CID 7505563.
- "Did you know DNA was used to uncover the origin of the House of Aisin Gioro?". Did You Know DNA... Retrieved 5 November 2020.
- Xue Y, Zerjal T, Bao W, Zhu S, Lim SK, Shu Q, et al. (December 2005). "Recent spread of a Y-chromosomal lineage in northern China and Mongolia". American Journal of Human Genetics. 77 (6): 1112–6. doi:10.1086/498583. PMC 1285168. PMID 16380921.
- "Asian Ancestry based on Studies of Y-DNA Variation: Part 3. Recent demographics and ancestry of the male East Asians – Empires and Dynasties". Genebase Tutorials. Archived from the original on November 25, 2013.
- Wei LH, Yan S, Yu G, Huang YZ, Yao DL, Li SL, et al. (March 2017). "Genetic trail for the early migrations of Aisin Gioro, the imperial house of the Qing dynasty". Journal of Human Genetics. The Japan Society of Human Genetics. 62 (3): 407–411. doi:10.1038/jhg.2016.142. PMID 27853133. S2CID 7685248.
- Yan S, Tachibana H, Wei LH, Yu G, Wen SQ, Wang CC (June 2015). "Y chromosome of Aisin Gioro, the imperial house of the Qing dynasty". Journal of Human Genetics. Nature Publishing Group on behalf of the Japan Society of Human Genetics (Japan). 60 (6): 295–8. arXiv:1412.6274. doi:10.1038/jhg.2015.28. PMID 25833470. S2CID 7505563.
- Wang CZ, Wei LH, Wang LX, Wen SQ, Yu XE, Shi MS, Li H (August 2019). "Relating Clans Ao and Aisin Gioro from northeast China by whole Y-chromosome sequencing". Journal of Human Genetics. Japan Society of Human Genetics. 64 (8): 775–780. doi:10.1038/s10038-019-0622-4. PMID 31148597. S2CID 171094135.
- Sukeyuki M, Kenichi S (2016-06-03). "The Origins of Japanese Culture Uncovered Using DNA ― What happens when we cut into the world of the Kojiki myths using the latest science". Discuss Japan-Japan Foreign Policy Forum. Retrieved 2019-01-21.
- Yang, Melinda A.; Fan, Xuechun; Sun, Bo; Chen, Chungyu; Lang, Jianfeng; Ko, Ying-Chin; Tsang, Cheng-hwa; Chiu, Hunglin; Wang, Tianyi; Bao, Qingchuan; Wu, Xiaohong (2020-07-17). "Ancient DNA indicates human population shifts and admixture in northern and southern China". Science. 369 (6501): 282–288. doi:10.1126/science.aba0909. ISSN 0036-8075. PMID 32409524.
- Boer, Elisabeth de; Yang, Melinda A.; Kawagoe, Aileen; Barnes, Gina L. (2020/ed). "Japan considered from the hypothesis of farmer/language spread". Evolutionary Human Sciences. 2. doi:10.1017/ehs.2020.7. ISSN 2513-843X. Check date values in:
|date=(help) - "'Jomon woman' helps solve Japan's genetic mystery | NHK WORLD-JAPAN News". NHK WORLD. Retrieved 2019-07-09.
- Nonaka I, Minaguchi K, Takezaki N (July 2007). "Y-chromosomal binary haplogroups in the Japanese population and their relationship to 16 Y-STR polymorphisms". Annals of Human Genetics. 71 (Pt 4): 480–95. doi:10.1111/j.1469-1809.2006.00343.x. hdl:10130/491. PMID 17274803. S2CID 1041367.
- Srithawong S, Srikummool M, Pittayaporn P, Ghirotto S, Chantawannakul P, Sun J, et al. (July 2015). "Genetic and linguistic correlation of the Kra-Dai-speaking groups in Thailand". Journal of Human Genetics. 60 (7): 371–80. doi:10.1038/jhg.2015.32. PMID 25833471. S2CID 21509343.
- 崎谷満『DNA・考古・言語の学際研究が示す新・日本列島史』(勉誠出版 2009年
- Robbeets M, Savelyev A (2017-12-21). Language Dispersal Beyond Farming. John Benjamins Publishing Company. ISBN 9789027264640.
- Takahashi R, Koibuchi R, Saeki F, Hagihara Y, Yoneda M, Adachi N, Nara T (2019). "Mitochondrial DNA analysis of the human skeletons excavated from the Shomyoji shell midden site, Kanagawa, Japan". Anthropological Science. 127 (1): 65–72. doi:10.1537/ase.190307.
- Hammer MF, Karafet TM, Park H, Omoto K, Harihara S, Stoneking M, Horai S (2006). "Dual origins of the Japanese: common ground for hunter-gatherer and farmer Y chromosomes". Journal of Human Genetics. 51 (1): 47–58. doi:10.1007/s10038-005-0322-0. PMID 16328082.
- Ochiai E, Minaguchi K, Nambiar P, Kakimoto Y, Satoh F, Nakatome M, Miyashita K, Osawa M (September 2016). "Evaluation of Y chromosomal SNP haplogrouping in the HID-Ion AmpliSeq™ Identity Panel". Legal Medicine. 22: 58–61. doi:10.1016/j.legalmed.2016.08.001. PMID 27591541.
- Hammer MF, Horai S (April 1995). "Y chromosomal DNA variation and the peopling of Japan". American Journal of Human Genetics. 56 (4): 951–62. PMC 1801189. PMID 7717406.
- Hashiyada M, Nata M, Nagashima T (2004). "Y-SNPs analysis in Japanese using liquid bead array technology". International Congress Series. 1261: 79–81. doi:10.1016/S0531-5131(03)01527-9.
- Sato Y, Shinka T, Ewis AA, Yamauchi A, Iwamoto T, Nakahori Y (2014). "Overview of genetic variation in the Y chromosome of modern Japanese males". Anthropological Science. 122 (3): 131–136. doi:10.1537/ase.140709. ISSN 0918-7960.
- Kim SH, Kim KC, Shin DJ, Jin HJ, Kwak KD, Han MS, Song JM, Kim W, Kim W (April 2011). "High frequencies of Y-chromosome haplogroup O2b-SRY465 lineages in Korea: a genetic perspective on the peopling of Korea". Investigative Genetics. 2 (1): 10. doi:10.1186/2041-2223-2-10. PMC 3087676. PMID 21463511.
- Xue Y, Zerjal T, Bao W, Zhu S, Shu Q, Xu J, Du R, Fu S, Li P, Hurles ME, Yang H, Tyler-Smith C (April 2006). "Male demography in East Asia: a north-south contrast in human population expansion times". Genetics. 172 (4): 2431–9. doi:10.1534/genetics.105.054270. PMC 1456369. PMID 16489223.
- Hammer MF, Horai S (April 1995). "Y chromosomal DNA variation and the peopling of Japan". American Journal of Human Genetics. 56 (4): 951–62. PMC 1801189. PMID 7717406.
- Underhill PA, Shen P, Lin AA, Jin L, Passarino G, Yang WH, et al. (November 2000). "Y chromosome sequence variation and the history of human populations". Nature Genetics. 26 (3): 358–61. doi:10.1038/81685. PMID 11062480. S2CID 12893406.
- Hammer MF, Karafet TM, Park H, Omoto K, Harihara S, Stoneking M, Horai S (2006). "Dual origins of the Japanese: common ground for hunter-gatherer and farmer Y chromosomes". Journal of Human Genetics. 51 (1): 47–58. doi:10.1007/s10038-005-0322-0. PMID 16328082.
- Travis J (February 1997). "Jomon Genes: Using DNA, researchers probe the genetic origins of modern japanese". Science News. 151 (7): 106–7. doi:10.2307/3980542. JSTOR 3980542.
- 238/744 = 32.0% O2b-SRY465 in a pool of all Japanese samples of Xue et al. (2006), Katoh et al. (2004), Han-Jun Jin et al. (2009), Nonaka et al. (2007), and all non-Ainu and non-Okinawan Japanese samples of Hammer et al. (2006).
- Jin HJ, Kwak KD, Hammer MF, Nakahori Y, Shinka T, Lee JW, Jin F, Jia X, Tyler-Smith C, Kim W (December 2003). "Y-chromosomal DNA haplogroups and their implications for the dual origins of the Koreans". Human Genetics. 114 (1): 27–35. doi:10.1007/s00439-003-1019-0. PMID 14505036. S2CID 1396796.
- Jin HJ, Tyler-Smith C, Kim W (2009). "The peopling of Korea revealed by analyses of mitochondrial DNA and Y-chromosomal markers". PLOS ONE. 4 (1): e4210. Bibcode:2009PLoSO...4.4210J. doi:10.1371/journal.pone.0004210. PMC 2615218. PMID 19148289.

- Katoh T, Munkhbat B, Tounai K, Mano S, Ando H, Oyungerel G, et al. (February 2005). "Genetic features of Mongolian ethnic groups revealed by Y-chromosomal analysis". Gene. 346: 63–70. doi:10.1016/j.gene.2004.10.023. PMID 15716011.
- 202/677 = 29.8% O2b-SRY465 in a pool of all ethnic Korean samples of Hammer et al. (2006), Xue et al. (2006), Katoh et al. (2004), Wook Kim et al. (2007), and Han-Jun Jin et al. (2009).
- Kim W, Yoo TK, Kim SJ, Shin DJ, Tyler-Smith C, Jin HJ, Kwak KD, Kim ET, Bae YS (January 2007). "Lack of association between Y-chromosomal haplogroups and prostate cancer in the Korean population". PLOS ONE. 2 (1): e172. Bibcode:2007PLoSO...2..172K. doi:10.1371/journal.pone.0000172. PMC 1766463. PMID 17245448.

- Nonaka I, Minaguchi K, Takezaki N (July 2007). "Y-chromosomal binary haplogroups in the Japanese population and their relationship to 16 Y-STR polymorphisms". Annals of Human Genetics. 71 (Pt 4): 480–95. doi:10.1111/j.1469-1809.2006.00343.x. hdl:10130/491. PMID 17274803. S2CID 1041367.
- The JPT sample is considered "as generally representative of the majority population in Japan". See Matsuda I. "Japanese in Tokyo, Japan – Population Description". Camden, NJ: Coriell Institute for Medical Research.
- Poznik GD, Xue Y, Mendez FL, Willems TF, Massaia A, Wilson Sayres MA, et al. (June 2016). "Punctuated bursts in human male demography inferred from 1,244 worldwide Y-chromosome sequences". Nature Genetics. 48 (6): 593–9. doi:10.1038/ng.3559. hdl:11858/00-001M-0000-002A-F024-C. PMC 4884158. PMID 27111036.
- Zheng H-X, Yan S, Qin Z-D, Wang Y, Tan J-Z, et al. (2011), "Major Population Expansion of East Asians Began before Neolithic Time: Evidence of mtDNA Genomes." PLoS ONE 6(10): e25835. doi:10.1371/journal.pone.0025835
- Yang X, Xu S (29 December 2011). "Identification of close relatives in the HUGO Pan-Asian SNP database". PLOS ONE. 6 (12): e29502. Bibcode:2011PLoSO...629502Y. doi:10.1371/journal.pone.0029502. PMC 3248454. PMID 22242128.
- Tian C, Kosoy R, Lee A, Ransom M, Belmont JW, Gregersen PK, Seldin MF (December 5, 2008). "Analysis of East Asia genetic substructure using genome-wide SNP arrays". PLOS ONE. 3 (12): e3862. Bibcode:2008PLoSO...3.3862T. doi:10.1371/journal.pone.0003862. PMC 2587696. PMID 19057645.
- "Pinning down Korean-ness through DNA". Korea JoongAng Daily (in Korean). Retrieved 2019-07-09.
- Matsumoto H (2009). "The origin of the Japanese race based on genetic markers of immunoglobulin G." Proceedings of the Japan Academy Series B. 85 (2): 69–82. Bibcode:2009PJAB...85...69M. doi:10.2183/pjab.85.69. PMC 3524296. PMID 19212099.
- Takeuchi F, Katsuya T, Kimura R, Nabika T, Isomura M, Ohkubo T, Tabara Y, Yamamoto K, Yokota M, Liu X, Saw WY, Mamatyusupu D, Yang W, Xu S, Teo YY, Kato N (2017). "The fine-scale genetic structure and evolution of the Japanese population". PLOS ONE. 12 (11): e0185487. Bibcode:2017PLoSO..1285487T. doi:10.1371/journal.pone.0185487. PMC 5665431. PMID 29091727.
- Chen A (2017-02-01). "Today's East Asians are very genetically similar to their ancient ancestors". The Verge. Retrieved 2019-04-17.
- "「縄文人」は独自進化したアジアの特異集団だった! : 深読み". 読売新聞オンライン (in Japanese). 2017-12-15. Retrieved 2019-04-17.
- Gakuhari T, Nakagome S, Rasmussen S, Allentoft M, Sato T, Korneliussen T, et al. (March 15, 2019) [2019]. "Jomon genome sheds light on East Asian population history" (PDF). bioRxiv: 3–5. Cite journal requires
|journal=(help) - Late Jomon male and female genome sequences from the Funadomari site in Hokkaido, Japan - Hideaki Kanzawa-Kiriyama, Department of Anthropology, National Museum of Nature and Science 2018/2019en
- Lee, Hasegawa, Sean, Toshikazu (April 2013). "Evolution of the Ainu Language in Space and Time".
In this paper, we reconstructed spatiotemporal evolution of 19 Ainu language varieties, and the results are in strong agreement with the hypothesis that a recent population expansion of the Okhotsk people played a critical role in shaping the Ainu people and their culture. Together with the recent archaeological, biological and cultural evidence, our phylogeographic reconstruction of the Ainu language strongly suggests that the conventional dual-structure model must be refined to explain these new bodies of evidence. The case of the Ainu language origin we report here also contributes additional detail to the global pattern of language evolution, and our language phylogeny might also provide a basis for making further inferences about the cultural dynamics of the Ainu speakers [44,45].
- 崎谷満『DNA・考古・言語の学際研究が示す新・日本列島史』(勉誠出版 2009年)(in Japanese)
- Schmidt, Seguchi (2014). "Jōmon culture and the peopling of the Japanese archipelago" (PDF).
In this respect, the biological identity of the Jomon is heterogeneous, and it may be indicative of diverse peoples who possibly belonged to a common culture, known as the Jomon
- Gakuhari, Takashi; Nakagome, Shigeki; Rasmussen, Simon; Allentoft, Morten; Sato, Takehiro; Korneliussen, Thorfinn; Chuinneagáin, Blánaid; Matsumae, Hiromi; Koganebuchi, Kae; Schmidt, Ryan; Mizushima, Souichiro (March 15, 2019) [2019]. "Jomon genome sheds light on East Asian population history" (PDF). bioRxiv: 3–5. Cite journal requires
|journal=(help) - Late Jomon male and female genome sequences from the Funadomari site in Hokkaido, Japan - Hideaki Kanzawa-Kiriyama, Department of Anthropology, National Museum of Nature and Science 2018/2019en
- Kim SH, Han MS, Kim W, Kim W (November 2010). "Y chromosome homogeneity in the Korean population". International Journal of Legal Medicine. 124 (6): 653–7. doi:10.1007/s00414-010-0501-1. PMID 20714743. S2CID 27125545.
- Reference Populations – Geno 2.0 Next Generation . (2017). The Genographic Project. Retrieved 15 May 2017, from link.
- Jin HJ, Tyler-Smith C, Kim W (2009-01-16). "The peopling of Korea revealed by analyses of mitochondrial DNA and Y-chromosomal markers". PLOS ONE. 4 (1): e4210. Bibcode:2009PLoSO...4.4210J. doi:10.1371/journal.pone.0004210. PMC 2615218. PMID 19148289.
- Balaresque P, Poulet N, Cussat-Blanc S, Gerard P, Quintana-Murci L, Heyer E, Jobling MA (October 2015). "Y-chromosome descent clusters and male differential reproductive success: young lineage expansions dominate Asian pastoral nomadic populations". European Journal of Human Genetics. 23 (10): 1413–22. doi:10.1038/ejhg.2014.285. PMC 4430317. PMID 25585703.
- Shi H, Dong YL, Wen B, Xiao CJ, Underhill PA, Shen PD, Chakraborty R, Jin L, Su B (September 2005). "Y-chromosome evidence of southern origin of the East Asian-specific haplogroup O3-M122". American Journal of Human Genetics. 77 (3): 408–19. doi:10.1086/444436. PMC 1226206. PMID 16080116.
- Shin DJ, Jin HJ, Kwak KD, Choi JW, Han MS, Kang PW, Choi SK, Kim W (October 2001). "Y-chromosome multiplexes and their potential for the DNA profiling of Koreans". International Journal of Legal Medicine. 115 (2): 109–17. doi:10.1007/s004140100248. PMID 11724428. S2CID 27739773.
- Park MJ, Lee HY, Kim NY, Lee EY, Yang WI, Shin KJ (January 2013). "Y-SNP miniplexes for East Asian Y-chromosomal haplogroup determination in degraded DNA". Forensic Science International. Genetics. 7 (1): 75–81. doi:10.1016/j.fsigen.2012.06.014. PMID 22818129.
- Kwon SY, Lee HY, Lee EY, Yang WI, Shin KJ (November 2015). "Confirmation of Y haplogroup tree topologies with newly suggested Y-SNPs for the C2, O2b and O3a subhaplogroups". Forensic Science International. Genetics. 19: 42–46. doi:10.1016/j.fsigen.2015.06.003. PMID 26103100.
- Wells RS, Yuldasheva N, Ruzibakiev R, Underhill PA, Evseeva I, Blue-Smith J, et al. (August 2001). "The Eurasian heartland: a continental perspective on Y-chromosome diversity". Proceedings of the National Academy of Sciences of the United States of America. 98 (18): 10244–9. Bibcode:2001PNAS...9810244W. doi:10.1073/pnas.171305098. PMC 56946. PMID 11526236.
- Tajima A, Hayami M, Tokunaga K, Juji T, Matsuo M, Marzuki S, Omoto K, Horai S (2004). "Genetic origins of the Ainu inferred from combined DNA analyses of maternal and paternal lineages". Journal of Human Genetics. 49 (4): 187–93. doi:10.1007/s10038-004-0131-x. PMID 14997363.
- Eugene Y. Park. (n.d.). Penn Arts & Sciences East Asian Languages and Civilizations. Retrieved January 24, 2018, from link. Archived 11 November 2017 at the Wayback Machine
- In a YouTube video which was published on May 18, 2015, from the 9:09 mark of the video to 9:56 mark of the video, Korea Foundation Associate Professor of History, Eugene Y. Park said, "Unlike the Jewish people, Jewish males with the traditionally priestly surnames such as the Levi, Levine, Cohen, and others, who claim that they're descended from Moses's brother, Aaron, and the Y-DNA analysis actually shows that the majority of the Jewish men with the, such surnames, descend from a single male, who lived about a hundred generations ago, around the time of the Exodus. In complete, in stark contrast, Y-DNA analysis shows that there's no correlation between Y-DNA haplotype or haplogroup, and a Korean person's surname or ancestral seat. It's fairly random."
- He M, Gitschier J, Zerjal T, de Knijff P, Tyler-Smith C, Xue Y (2009). "Geographical affinities of the HapMap samples". PLOS ONE. 4 (3): e4684. Bibcode:2009PLoSO...4.4684H. doi:10.1371/journal.pone.0004684. PMC 2649532. PMID 19259268.
- Kong QP, Yao YG, Liu M, Shen SP, Chen C, Zhu CL, Palanichamy MG, Zhang YP (October 2003). "Mitochondrial DNA sequence polymorphisms of five ethnic populations from northern China". Human Genetics. 113 (5): 391–405. doi:10.1007/s00439-003-1004-7. PMID 12938036. S2CID 6370358.
- Derenko M, Malyarchuk B, Grzybowski T, Denisova G, Dambueva I, Perkova M, et al. (November 2007). "Phylogeographic analysis of mitochondrial DNA in northern Asian populations". American Journal of Human Genetics. 81 (5): 1025–41. doi:10.1086/522933. PMC 2265662. PMID 17924343.
- Hwan Young Lee, Ji-Eun Yoo, Myung Jin Park, Ukhee Chung, Chong-Youl Kim, and Kyoung-Jin Shin, "East Asian mtDNA haplogroup determination in Koreans: Haplogroup-level coding region SNP analysis and subhaplogroup-level control region sequence analysis." Electrophoresis (2006). DOI 10.1002/elps.200600151.
- Hong SB, Kim KC, Kim W (2014). "Mitochondrial DNA haplogroups and homogeneity in the Korean population". Genes & Genomics. 36 (5): 583–590. doi:10.1007/s13258-014-0194-9. S2CID 16827252.
- Jeon S, Bhak Y, Choi Y, et al. (2020). "Korean Genome Project: 1094 Korean personal genomes with clinical information". Science Advances. 6 (22): eaaz7835. Bibcode:2020SciA....6.7835J. doi:10.1126/sciadv.aaz7835. PMC 7385432. PMID 32766443.
- Jin HJ, Choi JW, Shin DJ, Kim JM, Kim W (1999). "Distribution of length variation of the mtDNA 9‐bp motif in the intergenic COII/tRNALys region in East Asian populations". Korean Journal of Biological Sciences. 3 (4): 393–397. doi:10.1080/12265071.1999.9647513.
- Kim W, Shin DJ, Harihara S, Kim YJ (2000). "Y chromosomal DNA variation in east Asian populations and its potential for inferring the peopling of Korea". Journal of Human Genetics. 45 (2): 76–83. doi:10.1007/s100380050015. PMID 10721667.
- Kim JJ, Verdu P, Pakstis AJ, Speed WC, Kidd JR, Kidd KK (October 2005). "Use of autosomal loci for clustering individuals and populations of East Asian origin". Human Genetics. 117 (6): 511–9. doi:10.1007/s00439-005-1334-8. PMID 16028061. S2CID 15585215.
- Jung J, Kang H, Cho YS, Oh JH, Ryu MH, Chung HW, et al. (July 2010). "Gene flow between the Korean peninsula and its neighboring countries". PLOS ONE. 5 (7): e11855. Bibcode:2010PLoSO...511855J. doi:10.1371/journal.pone.0011855. PMC 2912326. PMID 20686617.
- "Han Chinese in Beijing, China [CHB]". Coriell Institute for Medical Research. 2018. Retrieved 27 February 2018.
- Siska V, Jones ER, Jeon S, Bhak Y, Kim HM, Cho YS, et al. (February 2017). "Genome-wide data from two early Neolithic East Asian individuals dating to 7700 years ago". Science Advances. 3 (2): e1601877. Bibcode:2017SciA....3E1877S. doi:10.1126/sciadv.1601877. PMC 5287702. PMID 28164156.
- Matsumoto H (2009). "The origin of the Japanese race based on genetic markers of immunoglobulin G". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 85 (2): 69–82. Bibcode:2009PJAB...85...69M. doi:10.2183/pjab.85.69. PMC 3524296. PMID 19212099.
- "DNA Match Solves Ancient Mystery". china.org.cn. Retrieved 2018-12-25.
- YFull MTree 1.01.5902 as of April 20, 2019
- Boris A Malyarchuk, Miroslava Derenko, Galina Denisova, Marcin Woźniak, Urszula Rogalla, Irina Dambueva, and Tomasz Grzybowski, "Y chromosome haplotype diversity in Mongolic-speaking populations and gene conversion at the duplicated STR DYS385a,b in haplogroup C3-M407." Journal of Human Genetics (2016) 61, 491–496; doi:10.1038/jhg.2016.14; published online 25 February 2016.
- Har'kov VN, Hamina KV, Medvedeva OF, Simonova KV, Eremina ER, Stepanov VA (February 2014). "[Gene pool of Buryats: clinal variability and territorial subdivision based on data of Y-chromosome markers]". Genetika (in Russian). 50 (2): 203–13. doi:10.1134/S1022795413110082. PMID 25711029. S2CID 15595963.
- Malyarchuk B, Derenko M, Denisova G, Khoyt S, Woźniak M, Grzybowski T, Zakharov I (December 2013). "Y-chromosome diversity in the Kalmyks at the ethnical and tribal levels". Journal of Human Genetics. 58 (12): 804–11. doi:10.1038/jhg.2013.108. PMID 24132124.
- Balinova N, Post H, Kushniarevich A, Flores R, Karmin M, Sahakyan H, et al. (September 2019). "Y-chromosomal analysis of clan structure of Kalmyks, the only European Mongol people, and their relationship to Oirat-Mongols of Inner Asia". European Journal of Human Genetics. 27 (9): 1466–1474. doi:10.1038/s41431-019-0399-0. PMC 6777519. PMID 30976109.
- Ilumäe 2016
- "Mongol Genetics – DNA of Mongolia's Khalkha Mongolians and others". www.khazaria.com. Retrieved 2018-12-25.
- Kolman CJ, Sambuughin N, Bermingham E (April 1996). "Mitochondrial DNA analysis of Mongolian populations and implications for the origin of New World founders". Genetics. 142 (4): 1321–34. PMC 1207128. PMID 8846908.
- Cheng B, Tang W, He L, Dong Y, Lu J, Lei Y, et al. (October 2008). "Genetic imprint of the Mongol: signal from phylogeographic analysis of mitochondrial DNA". Journal of Human Genetics. 53 (10): 905–913. doi:10.1007/s10038-008-0325-8. PMID 18769869.
European-prevalent haplogroups (HV, U, K, I, J) are 14% in western Xingjang Mongolian, 10% in Monglia, 8.4% in central Inner Mongolian samples, and only 2% in eastern Xin Barage Zuoqi County samples, showing decreasing frequencies from west to east
- Lu D, Lou H, Yuan K, Wang X, Wang Y, Zhang C, et al. (September 2016). "Ancestral Origins and Genetic History of Tibetan Highlanders". American Journal of Human Genetics. 99 (3): 580–594. doi:10.1016/j.ajhg.2016.07.002. PMC 5011065. PMID 27569548.
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, et al. (July 2010). "Sequencing of 50 human exomes reveals adaptation to high altitude". Science. 329 (5987): 75–8. Bibcode:2010Sci...329...75Y. doi:10.1126/science.1190371. PMC 3711608. PMID 20595611.
- Aldenderfer M, Yinong Z (March 2004). "The Prehistory of the Tibetan Plateau to the Seventh Century A.D.: Perspectives and Research from China and the West Since 1950". Journal of World Prehistory. 18 (1): 1–55. doi:10.1023/B:JOWO.0000038657.79035.9e. S2CID 154022638.
- Yuan B, Huang W, Zhang D (October 2007). "New evidence for human occupation of the northern Tibetan Plateau, China during the Late Pleistocene". Chinese Science Bulletin. 52 (19): 2675–2679. Bibcode:2007ChSBu..52.2675Y. doi:10.1007/s11434-007-0357-z. S2CID 93228078.
- Zhao M, Kong QP, Wang HW, Peng MS, Xie XD, Wang WZ, et al. (December 2009). "Mitochondrial genome evidence reveals successful Late Paleolithic settlement on the Tibetan Plateau". Proceedings of the National Academy of Sciences of the United States of America. 106 (50): 21230–5. Bibcode:2009PNAS..10621230Z. doi:10.1073/pnas.0907844106. PMC 2795552. PMID 19955425.
- Su, Bing, et al. (2000)
- Yunusbayev B, Metspalu M, Metspalu E, Valeev A, Litvinov S, Valiev R, et al. (April 2015). "The genetic legacy of the expansion of Turkic-speaking nomads across Eurasia". PLOS Genetics. 11 (4): e1005068. doi:10.1371/journal.pgen.1005068. PMC 4405460. PMID 25898006.
The origin and early dispersal history of the Turkic peoples is disputed, with candidates for their ancient homeland ranging from the Transcaspian steppe to Manchuria in Northeast Asia,
- Yunusbayev B, Metspalu M, Metspalu E, Valeev A, Litvinov S, Valiev R, et al. (April 2015). "The genetic legacy of the expansion of Turkic-speaking nomads across Eurasia". PLOS Genetics. 11 (4): e1005068. doi:10.1371/journal.pgen.1005068. PMC 4405460. PMID 25898006.
Thus, our study provides the first genetic evidence supporting one of the previously hypothesized IAHs to be near Mongolia and South Siberia.
- Lee J, Kuang S (Oct 2017). "A Comparative Analysis of Chinese Historical Sources and y-dna Studies with Regard to the Early and Medieval Turkic Peoples". Inner Asia. 19 (2): 197–239. doi:10.1163/22105018-12340089.
- Zerjal T, Wells RS, Yuldasheva N, Ruzibakiev R, Tyler-Smith C (September 2002). "A genetic landscape reshaped by recent events: Y-chromosomal insights into central Asia". American Journal of Human Genetics. 71 (3): 466–82. doi:10.1086/342096. PMC 419996. PMID 12145751.
- Kumar D (11 May 2012). Genomics and Health in the Developing World. Oxford, England: Oxford University Press. pp. 1265–1267. ISBN 978-0199705474.
- Alkan C, Kavak P, Somel M, Gokcumen O, Ugurlu S, Saygi C, Dal E, Bugra K, Güngör T, Sahinalp SC, Özören N, Bekpen C (November 2014). "Whole genome sequencing of Turkish genomes reveals functional private alleles and impact of genetic interactions with Europe, Asia and Africa". BMC Genomics. 15 (1): 963. doi:10.1186/1471-2164-15-963. PMC 4236450. PMID 25376095.
- Tyler-Smith, Chris; Zalloua, Pierre; Gasparini, Paolo; Comas, David; Xue, Yali; Mezzavilla, Massimo; Haber, Marc (2016). "Genetic evidence for an origin of the Armenians from Bronze Age mixing of multiple populations". European Journal of Human Genetics. 24 (6): 931–936. doi:10.1038/ejhg.2015.206. ISSN 1476-5438. PMC 4820045. PMID 26486470.
- Pakstis, Andrew J.; Gurkan, Cemal; Dogan, Mustafa; Balkaya, Hasan Emin; Dogan, Serkan; Neophytou, Pavlos I.; Cherni, Lotfi; Boussetta, Sami; Khodjet-El-Khil, Houssein; Ben Ammar ElGaaied, Amel; Salvo, Nina Mjølsnes; Janssen, Kirstin; Olsen, Gunn-Hege; Hadi, Sibte; Almohammed, Eida Khalaf; Pereira, Vania; Truelsen, Ditte Mikkelsen; Bulbul, Ozlem; Soundararajan, Usha; Rajeevan, Haseena; Kidd, Judith R.; Kidd, Kenneth K. (December 2019). "Genetic relationships of European, Mediterranean, and SW Asian populations using a panel of 55 AISNPs". European Journal of Human Genetics. 27 (12): 1885–1893. doi:10.1038/s41431-019-0466-6. ISSN 1476-5438. PMC 6871633. PMID 31285530.
- Hodoğlugil, U. U.; Mahley, R. W. (2012). "Turkish Population Structure and Genetic Ancestry Reveal Relatedness among Eurasian Populations". Annals of Human Genetics. 76 (2): 128–141. doi:10.1111/j.1469-1809.2011.00701.x. PMC 4904778. PMID 22332727.
- Bánfai Z, Melegh BI, Sümegi K, Hadzsiev K, Miseta A, Kásler M; et al. (2019). "Revealing the Genetic Impact of the Ottoman Occupation on Ethnic Groups of East-Central Europe and on the Roma Population of the Area". Front Genet. 10: 558. doi:10.3389/fgene.2019.00558. PMC 6585392. PMID 31263480.CS1 maint: multiple names: authors list (link)
- Ottoni, C.; Ricaut, F. O. X.; Vanderheyden, N.; Brucato, N.; Waelkens, M.; Decorte, R. (2011). "Mitochondrial analysis of a Byzantine population reveals the differential impact of multiple historical events in South Anatolia". European Journal of Human Genetics. 19 (5): 571–576. doi:10.1038/ejhg.2010.230. PMC 3083616. PMID 21224890.
- Ottoni, Claudio; Rasteiro, Rita; Willet, Rinse; Claeys, Johan; Talloen, Peter; Van De Vijver, Katrien; Chikhi, Lounès; Poblome, Jeroen; Decorte, Ronny (2016). "Comparing maternal genetic variation across two millennia reveals the demographic history of an ancient human population in southwest Turkey". Royal Society Open Science. 3 (2): 150250. Bibcode:2016RSOS....350250O. doi:10.1098/rsos.150250. PMC 4785964. PMID 26998313.
- Berkman, Ceren Caner (September 2006). Comparative Analyses For The Central Asian Contribution To Anatolian Gene Pool With Reference To Balkans (PDF) (PhD). Retrieved 30 October 2020.
- Cinnioglu, Cengiz; King, Roy; Kivisild, Toomas; Kalfoglu, Ersi; Atasoy, Sevil; Cavalleri, Gianpiero L.; Lillie, Anita S.; Roseman, Charles C.; Lin, Alice A.; Prince, Kristina; Oefner, Peter J.; Shen, Peidong; Semino, Ornella; Cavalli-Sforza, L. Luca; Underhill, Peter A. (2004). "Excavating Y-chromosome haplotype strata in Anatolia". Human Genetics. 114 (2): 127–48. doi:10.1007/s00439-003-1031-4. PMID 14586639. S2CID 10763736.
- Semino O, Magri C, Benuzzi G, Lin AA, Al-Zahery N, Battaglia V; et al. (2004). "Origin, diffusion, and differentiation of Y-chromosome haplogroups E and J: inferences on the neolithization of Europe and later migratory events in the Mediterranean area". Am J Hum Genet. 74 (5): 1023–34. doi:10.1086/386295. PMC 1181965. PMID 15069642.CS1 maint: multiple names: authors list (link)
- Shou, Wei-Hua; Qiao, En-Fa; Wei, Chuan-Yu; Dong, Yong-Li; Tan, Si-Jie; Shi, Hong; Tang, Wen-Ru; Xiao, Chun-Jie (2010). "Y-chromosome distributions among populations in Northwest China identify significant contribution from Central Asian pastoralists and lesser influence of western Eurasians". Journal of Human Genetics. 55 (5): 314–22. doi:10.1038/jhg.2010.30. PMID 20414255.
- Damgaard PB, Marchi N, Rasmussen S, Peyrot M, Renaud G, Korneliussen T, et al. (May 2018). "137 ancient human genomes from across the Eurasian steppes". Nature. 557 (7705): 369–374. Bibcode:2018Natur.557..369D. doi:10.1038/s41586-018-0094-2. PMID 29743675. S2CID 13670282.
- Yunusbayev B, Metspalu M, Metspalu E, Valeev A, Litvinov S, Valiev R, et al. (April 2015). "The genetic legacy of the expansion of Turkic-speaking nomads across Eurasia". PLOS Genetics. 11 (4): e1005068. doi:10.1371/journal.pgen.1005068. PMC 4405460. PMID 25898006.
- Zhang 2015.
- Ness 2014, p. 265.
- van Driem 2007.
- Chaubey 2011.
- Riccio ME, Nunes JM, Rahal M, Kervaire B, Tiercy JM, Sanchez-Mazas A (June 2011). "The Austroasiatic Munda population from India and Its enigmatic origin: a HLA diversity study". Human Biology. 83 (3): 405–35. doi:10.3378/027.083.0306. PMID 21740156. S2CID 39428816.
- Arunkumar 2015.
- "ASI-AAA"
- van Driem 2007, p. 7.
- Vilar M (2015). "DNA Reveals Unknown Ancient Migration Into India". National Geographic.
- Gutman A, Avanzati B. "Austroasiatic Languages". The Language Gulper.
- Liu D, Duong NT, Ton ND, Van Phong N, Pakendorf B, Van Hai N, Stoneking M (April 2020). "Extensive ethnolinguistic diversity in Vietnam reflects multiple sources of genetic diversity". Molecular Biology and Evolution. 37 (9): 2503–2519. doi:10.1093/molbev/msaa099. PMID 32344428.
Further reading
- He G, Wang Z, Guo J, Wang M, Zou X, Tang R, et al. (August 2020). "Inferring the population history of Tai-Kadai-speaking people and southernmost Han Chinese on Hainan Island by genome-wide array genotyping". European Journal of Human Genetics. 28 (8): 1111–1123. doi:10.1038/s41431-020-0599-7. PMID 32123326. S2CID 211729663.