IPEX syndrome
Immunodysregulation polyendocrinopathy enteropathy X-linked (or IPEX) syndrome is a rare disease linked to the dysfunction of the gene encoding transcription factor forkhead box P3 (FOXP3), widely considered to be the master regulator of the regulatory T cell lineage.[5][6] It leads to the dysfunction of CD4+ regulatory T-cells and the subsequent autoimmunity.[7] The disorder is one of the autoimmune polyendocrine syndromes and manifests with autoimmune enteropathy, psoriasiform or eczematous dermatitis, nail dystrophy, autoimmune endocrinopathies, and autoimmune skin conditions such as alopecia universalis and bullous pemphigoid.[7][2] Management for IPEX has seen limited success in treating the syndrome by bone marrow transplantation.[8]
| IPEX syndrome | |
|---|---|
| Other names | Autoimmune enteropathy type 1[1] |
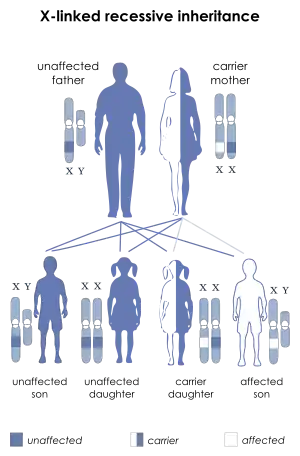 | |
| IPEX syndrome is inherited via X-linked recessive | |
| Specialty | Immunology |
| Symptoms | Lymphadenopathy[2] |
| Causes | FOXP3 gene mutation[1] |
| Diagnostic method | Family history, Genetic test[1] |
| Treatment | TPN(nutritional purpose), Cyclosporin A and FK506, Bone marrow transplant[3][4] |
Clinical manifestation of disease
The most representative criterion for diagnose of IPEX syndrome is autoimmune enteropathy. First symptoms of enteropathy begin in first day of life and they are characterized by diarrhea, vomiting, gastritis, ileus and colitis. The second hallmark is type 1 diabetes (T1D) and the worst complication of it is destruction of pancreas confirmed by histological examinations. Dermatitis is next sign and it can be presented in three forms: eczematiform (mainly atopic dermatitis), chthyosiform and psoriasiform or combinations of them. Other skin manifestations can include cheilitis, onychodystrophy and alopecia. Beside the three most significant triada of symptoms during this syndrom other not so typical symptoms include: thyroid and renal dysfunction, less number of thrombocytes and neutrophils, arthritis, splenomegaly, lymphadenopathy and infections. [9]
Family history of IPEX patients
IPEX patients are usually born with normal weight and length at term. Nevertheless, the first symptoms may present in the first days of life [10] some report cases labeled newborns with intrauterine growth retardation and evidence of meconium in the amniotic fluid.[11]
Triad of symptoms

A serious course of the disease is marked by a triad of symptoms: intractable diarrhea, T1D, and eczema.[12]
Clinical manifestations are including: enteropathy, skin manifestations, endocrinopathy, hematologic abnormalities, infections, autoimmune hemolytic anemia and food alergy. [12]
Genetics
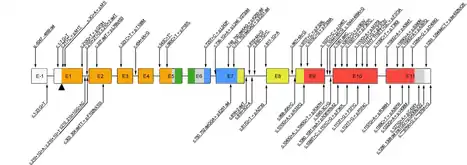
IPEX syndrome is inherited in males via an x-linked recessive manner, as the FOXP3 gene, whose cytogenetic location is Xp11.23, is involved in the mechanism of this condition.[5][6] FOXP3 gene has 12 exons and full reading open frame encodes 431 amino-acids. FOXP3 is a member of the FKH family of transcription factors and contains a proline‐rich (PRR) amino‐terminal domain, central zinc finger (ZF) and leucine zipper (LZ) domains important for protein–protein interactions, and a carboxyl‐terminal FKH domain required for nuclear localization and DNA‐binding activity. In humans exon 2 and 7 are spliced and excluded from protein. [9] Large number of mutations were found including single base substitutions, deletions, and splicing mutations. Consequence of malfunctioning FOXP3 expression leads to a defect in Treg production. Those patients are not having circulating CD41/CD251/FOXP31 T cells. Reduced expression of FOXP3 has been described, and these patients may express normal levels of dysfunctional protein which leads to mild symptoms later in life or during the neonatal period. In case of suspection on IPEX syndrom patients should do genetic testing although FOXP31 T cells are present in the periphery. [13]Mutation of FOXP3 leading to expression of malfunctioning protein is often localised in DNA-binding domain called the forkhead domain. The truncated protein can not bind to its binding-spot on the DNA and thus its function concerning T regulatory lymphocytes development and functioning is impaired. The absence or dysfunction of regulatory T cells is the cause of autoimmune symptoms.[14]
Data from 2018 describes over 70 mutations in FOXP3 gene leading to IPEX syndrome. Nonetheless, this number is still changing with new cases and discoveries coming.[15] For example in 2010 there were only 20 mutations of FOXP3 known in the literature.[14]
FOXP3 pathways
FOXP3 can function as both a repressor and a trans‐activator of Treg cells depending on interactions with other proteins. FOXP3 express it's activity by controlling transcription as well as influencing epigenetic changes and posttranscriptional modifications. N‐terminal repressor domain of FOXP3 can change transcription or epigenetic regulation of Treg cells. Transcription activity is changing by interacting of N-terminal domain with Eos, which associates with CtBP1 and forms a corepressor complex that binds the IL2 promoter and enables FOXP3 to repress IL2 transcription in Treg cells. FOXP3 forms complexes with histone deacetylase (HDAC) seven, HDAC9, and the histone acetyl transferase TIP60 converts epigenetic activity of Treg cells. N‐terminal domain of FOXP3 also can to interact with RORγ and RORα antagonizing the action of these transcription factors, thereby inhibiting TH17 cell differentiation. FOXP3 is linked with TCR signaling by donwnstream transcription factors. All of this findings verify importance of FOXP3 in regulation of transcriptional activity/repression of Treg cells.[9]
Mechanism
This autoimmunity called IPEX is an attack from the body's own immune system against the body's own tissues and organs.[4] Early age onset of this disease in males causes severe enlargement of the secondary lymphoid organs, and insulin dependent diabetes
This condition indicates the loss of CD4+ CD25+ T regulatory cells, and express the transcription factor Foxp3. Foxp3 decrease is a consequence of unchecked T cell activation, which is secondary to loss of regulatory T cells.[16]
Causes of death include haemorrhage, sepsis, Intractable diarrhoea and diabetic complications [8]
Diagnosis
Early detection of the disease is crucial because mortality is on high level without treatment.[13] The diagnosis of immunodysregulation polyendocrinopathy enteropathy X-linked syndrome is consistent with the following criteria:[1][4]
- Clinical triad
- Family history
- Laboratory findings: elevated serum concentration of IgE, eosinophilia, autoimmune anemia and decresed number of FOXP3 Treg cells.
- Genetic testing: single-gene testing and multigene panel.
Treatment
In terms of treatment the following are done to manage the IPEX syndrome in those affected individuals (corticosteroids are the first treatment that is used):[4][3]
- TPN (nutritional purpose)
- Cyclosporin A and FK506
- Sirolimus (should FK506 prove non-effective)
- Granulocyte colony stimulating factor
- Bone marrow transplant
- Rituximab
Research
In non-human research that has been conducted there is as well a special mouse model simulating the development and progression of the IPEX syndrome. The model mice are called "scurfy mice" and they have had 2 base pairs inserted within the Foxp3 gene. This leads to a frameshift mutation in Foxp3 gene and the expressed protein is truncated, causing functional deficiency of Treg cells. Consequently, autoreactive CD4+T cells and inflammatory cells are causing tissue damaging. Beside CD4+T cells to inflammation disorder contribute B cells by producing autoantibodies like antinuclear antibodies.[17] The mice suffer from enlarged spleen and lymph nodes, redness in eyes, and skin abnormalities. The mice as well suffer from immunity problems and after approximately 3 weeks they die.[15]
References
- "Orphanet: Immune dysregulation polyendocrinopathy enteropathy X linked syndrome". www.orpha.net. Retrieved 2017-04-18.
- "Immunodysregulation, polyendocrinopathy and enteropathy X-linked | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Retrieved 2017-04-16.
- Eisenbarth GS (2010-12-13). Immunoendocrinology: Scientific and Clinical Aspects. Springer Science & Business Media. pp. 129–138. ISBN 9781603274784.
- Hannibal M, Torgerson T (1993-01-01). "IPEX Syndrome". In RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, Bird TD, Ledbetter N, Mefford H (eds.). GeneReviews. Seattle (WA): University of Washington, Seattle. PMID 20301297.update 2011
- "IPEX syndrome". Genetics Home Reference. Retrieved 2017-04-16.
- "FOXP3 gene". Genetics Home Reference. Retrieved 2017-04-16.
- Rapini RP, Bolognia JL, Jorizzo JL (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. p. 72. ISBN 978-1-4160-2999-1.
- Wildin RS, Smyk-Pearson S, Filipovich AH (August 2002). "Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome". Journal of Medical Genetics. 39 (8): 537–45. doi:10.1136/jmg.39.8.537. PMC 1735203. PMID 12161590.
- Bacchetta, Rosa; Barzaghi, Federica; Roncarolo, Maria-Grazia (2018). "From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation". Annals of the New York Academy of Sciences. 1417 (1): 5–22. doi:10.1111/nyas.13011. ISSN 1749-6632.
- Barzaghi F, Passerini L, Bacchetta R (2012). "Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity". Frontiers in Immunology. 3: 211. doi:10.3389/fimmu.2012.00211. PMID 23060872.
- Xavier-da-Silva MM, Moreira-Filho CA, Suzuki E, Patricio F, Coutinho A, Carneiro-Sampaio M (February 2015). "Fetal-onset IPEX: report of two families and review of literature". Clinical Immunology. 156 (2): 131–40. doi:10.1016/j.clim.2014.12.007. PMID 25546394.
- Park JH, Lee KH, Jeon B, Ochs HD, Lee JS, Gee HY, et al. (June 2020). "Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome: A systematic review". Autoimmunity Reviews. 19 (6): 102526. doi:10.1016/j.autrev.2020.102526. PMID 32234571.
- Kitcharoensakkul, Maleewan; Cooper, Megan A. (2020-01-01), Rose, Noel R.; Mackay, Ian R. (eds.), "Chapter 28 - Autoimmunity in Primary Immunodeficiency Disorders", The Autoimmune Diseases (Sixth Edition), Academic Press, pp. 513–532, doi:10.1016/b978-0-12-812102-3.00028-2, ISBN 978-0-12-812102-3, retrieved 2021-01-29
- Michels AW, Gottlieb PA (May 2010). "Autoimmune polyglandular syndromes". Nature Reviews. Endocrinology. 6 (5): 270–7. doi:10.1038/nrendo.2010.40. PMID 20309000. S2CID 20395564.
- Bacchetta R, Barzaghi F, Roncarolo MG (April 2018). "From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation". Annals of the New York Academy of Sciences. 1417 (1): 5–22. doi:10.1111/nyas.13011. PMID 26918796.
- Verbsky JW, Chatila TA (December 2013). "Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases". Current Opinion in Pediatrics. 25 (6): 708–14. doi:10.1097/MOP.0000000000000029. PMC 4047515. PMID 24240290.
- Yilmaz OK, Haeberle S, Zhang M, Fritzler MJ, Enk AH, Hadaschik EN (2019). "Scurfy Mice Develop Features of Connective Tissue Disease Overlap Syndrome and Mixed Connective Tissue Disease in the Absence of Regulatory T Cells". Frontiers in Immunology. 10: 881. doi:10.3389/fimmu.2019.00881. PMC 6491778. PMID 31068947.
Further reading
- Bacchetta R, Barzaghi F, Roncarolo MG (April 2018). "From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation". Annals of the New York Academy of Sciences. 1417 (1): 5–22. doi:10.1111/nyas.13011. PMID 26918796.
- Barzaghi F, Passerini L, Bacchetta R (1 January 2012). "Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity". Frontiers in Immunology. 3: 211. doi:10.3389/fimmu.2012.00211. PMC 3459184. PMID 23060872.
- Elzouki AY, Harfi HA, Nazer H, Stapleton FB, Oh W, Whitley RJ (2012-01-10). Textbook of Clinical Pediatrics. Springer Science & Business Media. ISBN 9783642022029.
External links
| Classification | |
|---|---|
| External resources |