Lapaquistat
Lapaquistat (TAK-475) is a cholesterol-lowering drug candidate that was abandoned before being marketed.
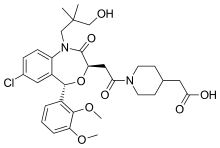 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C31H39ClN2O8 |
| Molar mass | 603.11 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Unlike statins, which inhibit HMG-CoA reductase, lapaquistat metabolites inhibit squalene synthase, which is further downstream in the synthesis of cholesterol. It is hoped that side effects can be reduced by not disturbing the mevalonate pathway, which is important for other biochemical molecules besides cholesterol. However, there is increasing evidence that statins (which inhibit the mevalonate pathway) may be clinically useful because they affect these other molecules (including protein prenylation).[1]
On March 28, 2008, Takeda halted further development of lapaquistat.[2] While effective at lowering low-density lipoprotein cholesterol in a dose-dependent manner, development of the drug was ceased due to observations in clinical trials that it might cause liver damage in the high dose trial groups.[3] Data from knockout mouse studies suggests that accumulation of high levels of the metabolic substrate of squalene synthase and derivatives thereof account for the liver toxicity of squalene synthase inhibitors,[4] and efforts to mitigate this substrate accumulation would likely be necessary for clinical success of a squalene synthase inhibitor [5]
References
- Greenwood J, Steinman L, Zamvil SS (May 2006). "Statin therapy and autoimmune disease: from protein prenylation to immunomodulation". Nat. Rev. Immunol. 6 (5): 358–70. doi:10.1038/nri1839. PMC 3842637. PMID 16639429.
- Takeda Pharmaceutical Company Limited press release - Discontinuation of Development of TAK-475, A Compound for Treatment of Hypercholesterolemia
- Stein, Evan; et al. (April 25, 2011). "Lapaquistat Acetate, Development of a Squalene Synthase Inhibitor for the Treatment of Hypercholesterolemia". Circulation. 123 (18): 1974–1985. doi:10.1161/CIRCULATIONAHA.110.975284. PMID 21518985.
- Nagashima S; Yagyu H; Tozawa R; Tazoe F; Takahashi M; Kitamine T; et al. (2015). "Plasma cholesterol-lowering and transient liver dysfunction in mice lacking squalene synthase in the liver". J Lipid Res. 56 (5): 998–1005. doi:10.1194/jlr.M057406. PMC 4409289. PMID 25755092.
- Wasko BM, Smits JP, Shull LW, Wiemer DF, Hohl RJ (2011). "A novel bisphosphonate inhibitor of squalene synthase combined with a statin or a nitrogenous bisphosphonate in vitro". J Lipid Res. 52 (11): 1957–64. doi:10.1194/jlr.M016089. PMC 3196227. PMID 21903868.
Further reading
- Davidson MH (January 2007). "Squalene synthase inhibition: a novel target for the management of dyslipidemia". Curr Atheroscler Rep. 9 (1): 78–80. doi:10.1007/BF02693932. PMID 17169251. S2CID 28176904.