Methionine sulfoxide
Methionine sulfoxide is the organic compound with the formula CH3S(O)CH2CH2CH(NH2)CO2H. It is an amino acid that occurs naturally although it is formed post-translationally.
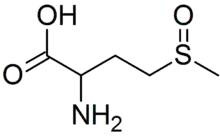 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-4-(methylsulfinyl)butanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.057.891 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H11NO3S | |
| Molar mass | 165.21 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oxidation of the sulfur of methionine results in methionine sulfoxide or methionine sulfone. The sulfur-containing amino acids methionine and cysteine are more easily oxidized than the other amino acids.[1] Unlike oxidation of other amino acids, the oxidation of methionine can be reversed by enzymatic action, specifically by enzymes in the methionine sulfoxide reductase family of enzymes. The three known methionine sulfoxide reductases are MsrA, MsrB, and fRmsr.[1] Oxidation of methionine results in a mixture of the two diastereomers methionine-S-sulfoxide and methionine-R-sulfoxide, which are reduced by MsrA and MsrB, respectively.[2] MsrA can reduce both free and protein-based methionine-S-sulfoxide, whereas MsrB is specific for protein-based methionine-R-sulfoxide. fRmsr, however, catalyzes the reduction of free methionine-R-sulfoxide.[1] Thioredoxin serves to recycle by reduction some of the methionine sulfoxide reductase family of enzymes, whereas others can be reduced by metallothionein.[3]
Biochemical Function
Methionine sulfoxide (MetO), the oxidized form of the amino acid methionine (Met), increases with age in body tissues, which is believed by some to contribute to biological ageing.[4][5] Oxidation of methionine residues in tissue proteins can cause them to misfold or otherwise render them dysfunctional.[4] Uniquely, the methionine sulfoxide reductase (Msr) group of enzymes act with thioredoxin to catalyze the enzymatic reduction and repair of oxidized methionine residues.[4] Moreover, levels of methionine sulfoxide reductase A (MsrA) decline in aging tissues in mice and in association with age-related disease in humans.[4] There is thus a rationale for thinking that by maintaining the structure, increased levels or activity of MsrA might retard the rate of aging.
Indeed, transgenic Drosophila (fruit flies) that overexpress methionine sulfoxide reductase show extended lifespan.[6] However, the effects of MsrA overexpression in mice were ambiguous.[7] MsrA is found in both the cytosol and the energy-producing mitochondria, where most of the body's endogenous free radicals are produced. Transgenically increasing the levels of MsrA in either the cytosol or the mitochondria had no significant effect on lifespan assessed by most standard statistical tests, and may possibly have led to early deaths in the cytosol-specific mice, although the survival curves appeared to suggest a slight increase in maximum (90%) survivorship, as did analysis using Boschloo's Exact test, a binomial test designed to test greater extreme variation.[7]
The oxidation of methionine serves as a switch that deactivates certain protein activities such as E.coli ribosomal protein, L12.[8] Proteins with great amount of methionine residues tend to exist within the lipid bilayer as methionine is one of the most hydrophobic amino acids. Those methionine residues that are exposed to the aqueous exterior thus are vulnerable to oxidation.2 The oxidized residues tend to be arrayed around the active site and may guard access to this site by reactive oxygen species. Once oxidized, the met(o) residues are reduced back to methionine by the enzyme methionine sulfoxide reductase.3 Thus, an oxidation–reduction cycle occurs in which exposed methionine residues are oxidized (e.g., by H2O2) to methionine sulfoxide residues, which are subsequently reduced.[9]
Methionine(protein)+ H2O2→ Methionine Sulfoxide(protein)+ H2O
Methionine Sulfoxide(protein)+ NADPH+H+→ Methionine(protein)+ NADP++H2O
See also
- MSRA (gene)
- MSRB2 enzyme
- SEPX1 enzyme
References
- Lee BC, Dikiy A, Kim HY, Gladyshev VN (2009). "Functions and evolution of selenoprotein methionine sulfoxide reductases". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (11): 1471–1477. doi:10.1016/j.bbagen.2009.04.014. PMC 3062201. PMID 19406207.
- Kim HY, Gladyshev VN (2004). "Methionine sulfoxide reduction in mammals: characterization of methionine-R-sulfoxide reductases". Molecular Biology of the Cell. 15 (3): 1055–1064. doi:10.1091/mbc.E03-08-0629. PMC 363075. PMID 14699060.
- Sagher D, Brunell D, Hejtmancik JF, Kantorow M, Brot N, Weissbach H (2006). "Thionein can serve as a reducing agent for the methionine sulfoxide reductases". Proceedings of the National Academy of Sciences of the United States of America. 103 (23): 8656–8661. Bibcode:2006PNAS..103.8656S. doi:10.1073/pnas.0602826103. PMC 1592241. PMID 16735467.
- Stadtman ER, Van Remmen H, Richardson A, Wehr NB, Levine RL (2005). "Methionine oxidation and aging". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1703 (2): 135–140. doi:10.1016/j.bbapap.2004.08.010. PMID 15680221.
- Shringarpure R, Davies KJ (2002). "Protein turnover by the proteasome in aging and disease". Free Radical Biology & Medicine. 32 (11): 1084–1089. doi:10.1016/S0891-5849(02)00824-9. PMID 12031893.
- Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T (2002). "High-quality life extension by the enzyme peptide methionine sulfoxide reductase". Proceedings of the National Academy of Sciences of the United States of America. 99 (5): 2748–2753. Bibcode:2002PNAS...99.2748R. doi:10.1073/pnas.032671199. PMC 122419. PMID 11867705.
- Salmon AB, Kim G, Liu C, Wren JD, Georgescu C, Richardson A, Levine RL (December 2016). "Effects of transgenic methionine sulfoxide reductase A (MsrA) expression on lifespan and age-dependent changes in metabolic function in mice". Redox Biol. 10: 251–256. doi:10.1016/j.redox.2016.10.012. PMC 5099276. PMID 27821326.
- Brot, N; Weissbach, L; Werth, J; Weissbach, H (April 1981). "Enzymatic reduction of protein-bound methionine sulfoxide". Proceedings of the National Academy of Sciences of the United States of America. 78 (4): 2155–8. Bibcode:1981PNAS...78.2155B. doi:10.1073/pnas.78.4.2155. PMC 319302. PMID 7017726.
- Levine, RL; Mosoni, L; Berlett, BS; Stadtman, ER (Dec 24, 1996). "Methionine residues as endogenous antioxidants in proteins". Proceedings of the National Academy of Sciences of the United States of America. 93 (26): 15036–40. Bibcode:1996PNAS...9315036L. doi:10.1073/pnas.93.26.15036. PMC 26351. PMID 8986759.
