Pristinamycin
Pristinamycin (INN), also spelled pristinamycine, is an antibiotic used primarily in the treatment of staphylococcal infections, and to a lesser extent streptococcal infections. It is a streptogramin group antibiotic, similar to virginiamycin, derived from the bacterium Streptomyces pristinaespiralis. It is marketed in Europe by Sanofi-Aventis under the trade name Pyostacine.
 | |
 | |
| Combination of | |
|---|---|
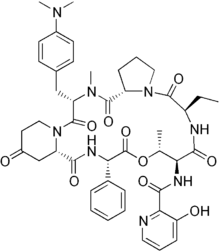
| Pristinamycin IA | antibiotic |
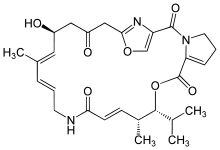
| Pristinamycin IIA | antibiotic |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a603007 |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| | |
Pristinamycin is a mixture of two components that have a synergistic antibacterial action. Pristinamycin IA is a macrolide, and results in pristinamycin's having a similar spectrum of action to erythromycin. Pristinamycin IIA (streptogramin A) is a depsipeptide.[1] PI and PII are coproduced by S. pristinaespiralis in a ratio of 30:70. Each compound binds to the bacterial 50 S ribosomal subunit and inhibits the elongation process of the protein synthesis, thereby exhibiting only a moderate bacteriostatic activity. However, the combination of both substances acts synergistically and leads to a potent bactericidal activity that can reach up to 100 times that of the separate components.
The pristinamycin biosynthetic gene cluster is the largest antibiotic supercluster known so far, with a size of ~210 kb, wherein the PI and PII biosynthetic genes are not clustered individually but are scattered across the complete sequence region.[2] Furthermore, this biosynthetic gene region is interrupted by a cryptic type II PKS gene cluster.
Clinical use
Despite the macrolide component, it is effective against erythromycin-resistant staphylococci and strepcococci.[3][4] It is active against methicillin-resistant Staphylococcus aureus (MRSA). Its usefulness for severe infections, however, may be limited by the lack of an intravenous formulation owing to its poor solubility.[5] Nevertheless, it is sometimes used as an alternative to rifampicin+fusidic acid or linezolid for the treatment of MRSA.
The lack of an intravenous formulation led to the development of the pristinamycin-derivative quinupristin/dalfopristin (i.e., Synercid), which may be administered intravenously for more severe MRSA infections.
See also
Footnotes
- Hamilton-Miller J (1991). "From foreign pharmacopoeias: 'new' antibiotics from old?". J Antimicrob Chemother. 27 (6): 702–5. doi:10.1093/jac/27.6.702. PMID 1938680.
- Mast Y, Weber T, Gölz M, Ort-Winklbauer R, Gondran A, Wohlleben W, Schinko E (2010) Characterization of the ‘pristinamycin supercluster’ of Streptomyces pristinaespiralis. Microbial Biotechnology. doi:10.1111/j.1751-7915.2010.00213.x
- Weber P (2001). "[Streptococcus pneumoniae: lack of emergence of pristinamycin resistance]". Pathol Biol (Paris). 49 (10): 840–5. doi:10.1016/S0369-8114(01)00255-3. PMID 11776696.
- Leclercq R, Soussy C, Weber P, Moniot-Ville N, Dib C (2003). "[In vitro activity of the pristinamycin against the isolated staphylococci in the french hospitals in 1999-2000]". Pathol Biol (Paris). 51 (7): 400–4. doi:10.1016/S0369-8114(03)00054-3. PMID 12948760.
- Sean C. Sweetman, ed. (November 30, 2004). Martindale: The complete drug reference (34th ed.). London: Pharmaceutical Press. ISBN 0-85369-550-4.