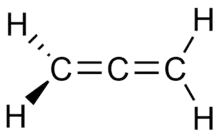Propadiene
Propadiene (/proʊpəˈdaɪiːn/) or allene (/ˈæliːn/) is the organic compound with the formula H2C=C=CH2. It is the simplest allene i.e. a compound with two adjacent carbon double bonds.[3] As a constituent of MAPP gas, it has been used as a fuel for specialized welding.
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propa-1,2-diene[1] | |||
| Other names
Allene[1] Propadiene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1730774 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.670 | ||
| EC Number |
| ||
| 860 | |||
| MeSH | Propadiene | ||
PubChem CID |
|||
| UNII | |||
| UN number | 2200 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H4 | |||
| Molar mass | 40.065 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Melting point | −136 °C (−213 °F; 137 K) | ||
| Boiling point | −34 °C (−29 °F; 239 K) | ||
| log P | 1.45 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |   [2] [2] | ||
| GHS Signal word | Danger | ||
| H220, H280[2] | |||
| P210, P377, P381, P410+403[2] | |||
| NFPA 704 (fire diamond) | |||
| Explosive limits | 13% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production and equilibrium with methylacetylene
Allene exists in equilibrium with methylacetylene (propyne) and the mixture is sometimes called MAPD for methylacetylene-propadiene:
- H3CC≡CH H2C=C=CH2
for which Keq = 0.22 at 270 °C or 0.1 at 5 °C.
MAPD is produced as a side product, often an undesirable one, of dehydrogenation of propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene.[4]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 375. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The name allene, for CH2=C=CH2, is retained for general nomenclature only; substitution is allowed, but not by alkyl or any other group that extends the carbon chain, nor characteristic groups expressed by suffixes. The systematic name, propa-1,2-diene, is the preferred IUPAC name.
- Record of Allene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 17 November 2020.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "allenes". doi:10.1351/goldbook.A00238
- Klaus Buckl, Andreas Meiswinkel "Propyne" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.m22_m01
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.