Quercus infectoria
Quercus infectoria, the Aleppo oak, is a species of oak, bearing galls that have been traditionally used for centuries in Asia medicinally. Manjakani is the name used in Malaysia for the galls; these have been used for centuries in softening leather and in making black dye and ink.[3] In India the galls are called majuphal among many other names.
| Aleppo oak | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Fagales |
| Family: | Fagaceae |
| Genus: | Quercus |
| Subgenus: | Quercus subg. Quercus |
| Section: | Quercus sect. Cerris |
| Species: | Q. infectoria |
| Binomial name | |
| Quercus infectoria Oliv. 1801 | |
| Synonyms[1][2] | |
|
List
| |
Distribution
Quercus infectoria is indigenous to parts of southern Europe (Greece and the East Aegean Islands) and the Middle East (Turkey, Cyprus, Iran, Iraq, Lebanon, Syria and Israel).[4]
Description
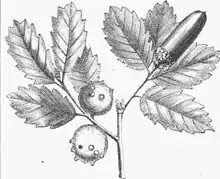
Quercus infectoria is a small tree native of Greece and Asia Minor, with one to two metres (four to six feet) in height. The stems are crooked, shrubby looking with smooth and bright-green leaves borne on short petioles of 3 to 4 cm (1 to 1.5 inches) long. The leaves are bluntly mucronate, rounded, smooth, unequal at the base and shiny on the upper side.
The galls arise on young branches of the Quercus infectoria tree when gall wasps[5] sting the oak tree and deposit their larvae the chemical reaction causes an abnormality in the oak tree causing hard balls to be formed. They are corrugated in appearance.
Uses
Quercus infectoria can be used as a thickener in stews or mixed with cereals for making bread.[6]
Also known as Majuphal in Indian traditional medicine, manjakani has been used as dental powder and in the treatment of toothache and gingivitis.[7][8]
The so-called "Aleppo tannin" is Tannic acid gained from Aleppo oak galls, which displays unique chemical properties essential in the preparation of gold sols (colloids) used as markers in Immunocytochemistry.[9] [10]
Nowadays, gallnut extracts are also widely used in pharmaceuticals, food and feed additives, dyes, inks, and metallurgy.
Constituents
The galls from Quercus infectoria contain the highest naturally occurring level of tannin, approx. 50–70%,[11] syringic acid, β-sitosterol, amentoflavone, hexamethyl ether, isocryptomerin, methyl betulate, methyl oleanate and hexagalloyl glucose.[12][13][11][14] They also contain 2-4% each of gallic and ellagic acid that are polymerized to make tannins.[15][16][17] Tannins have been used for hundreds of years for medical purposes and are currently indispensable in dermatology [18] and have been used for tanning of leather.
Tannins from the nutgalls
Tannins comprise a large group of natural products widely distributed in the plant kingdom. They have a great structural diversity, but are usually divided into two basic groups: the hydrolyzable type and the condensed type. Hydrolyzable tannins include the commonly occurring gallic and ellagic acid contained in the nut galls.
Hydrolyzable tannins are present in many different plant species but are found in particularly high concentrations in nut galls growing on Rhus semialata (Chinese and Korean gallotannins) and Quercus infectoria (Turkish and Chinese gallotannins), the seedpods of Caesalpinia spinosa (Tara tannins), and the fruits of Terminalia chebula. The gallic and ellagic acid hydrolyzable tannins react with proteins to produce typical tanning effects; medicinally, this is important to topically treat inflamed or ulcerated tissues. They also contribute to most of the astringent property of manjakani and in small insignificant doses, are great for skin whitening and killing microorganisms.
Although both types of tannin have been used to treat diseases in traditional medicine, the hydrolyzable tannins have long been considered official medicinal agents in Europe and North America. They have been included in many pharmacopoeias, in the older editions in particular, and are specifically referred to as tannic acid. These were recommended for treatment of inflammation and ulceration, including topical application for skin diseases and internal use for intestinal ulceration and diarrhea. In China, tannin-containing substances, such as galls, pomegranate rinds, and terminalia fruits, are used in several medicinal preparations.
Pharmacology
The galls of Quercus infectoria have also been pharmacologically documented to possess astringent, antidiabetic,[19] antitremorine, local anaesthetic,[20] antiviral,[21] antibacterial,[22] antifungal,[23] larvicidal [24] and anti-inflammatory [25] activities. The main constituents found in the galls of Quercus infectoria are tannin (50-70%) and small amount of free gallic acid and ellagic acid.[26][27][28]
The wide range of pharmacological activities of this plant might support the efficacy of extract preparation of Quercus infectoria that are widely used in Malaysia for treating many kinds of health problems since many decades ago. The nutgalls have been pharmacologically documented on their antiamoebic,[29] anticariogenic [30] and anti-inflammatory [31] activities, to treat skin infections and gastrointestinal disorders.[32][33][34][35]
Uses of the galls
Nowadays, gall nut extracts are widely used in pharmaceuticals, medical laboratory techniques as well as inks which use "Aleppo tannin",[36] food and feed additives, dyes, and metallurgy.
Tanning
Tannin, a substance contained in the galls of the Quercus infectoria, has been used for centuries for the tanning of leather.
Medical laboratory techniques
The so-called "Aleppo tannin" is tannic acid gained from Aleppo pine galls, which displays unique chemical properties essential in the preparation of gold sols (colloids) used as markers in immunocytochemistry.[37] [38]
Teeth and gum remedy
Also known as majuphal in Indian traditional medicine, manjakani has been used as dental powder and in the treatment of toothache and gingivitis.[39][40]
Uterine and vaginal therapy
The galls, locally known as manjakani in Malaysia, are used in combination with other herbs as drinking remedies by women after childbirth to restore the elasticity of the uterine wall, and in many vaginal tightening products.[41] The extract of manjakani was claimed by the Malay Kelantanese to be highly beneficial for postpartum women. Hazardous effects of the extract were not reported so far. In addition, the Arabs, Persians, Indians, Malays and Chinese have traditionally used the galls after childbirth to treat vaginal discharge and related postpartum infections.
Gallery
 Autumn foliage, Izmir, Turkey
Autumn foliage, Izmir, Turkey leaves with galls
leaves with galls leaf with galls
leaf with galls gall
gall gall
gall
See also
References
- "Quercus infectoria G.Olivier". World Checklist of Selected Plant Families (WCSP). Royal Botanic Gardens, Kew – via The Plant List.
- "Quercus infectoria Olivier". Tropicos. Missouri Botanical Garden.
- Kottakkal AVS (1995). The Indian Medicinal Plants.
- T. K. Lim 2012. Quercus infectoria. Edible Medicinal And Non-Medicinal Plants, pages 16-26
- Muhamad Z; Mustafa AM (1994). Traditional Malay Medicinal Plants. Kuala Lumpur: Penerbit Fajar Bakti Sdn Bhd.
- Stashia Eleaness; Rosland Abel (2013). "1". In Universiti Teknologi Malaysia (ed.). The extraction of essential oil from Quercus infectoria (Manjakani) galls using supercritical carbon dioxide pressure swing technique. Faculty of Chemical Engineering.
- Kottakkal AVS. (1995). Indian Medicinal Plants. 4. Orient Longman Ltd.
- Bhattacharjee SK. (2001). Handbook of Medicinal Plants. India: Pointer Publishers.
- Frank Mayer (1988). Electron Microscopy in Microbiology. 20. London: Academic Press. p. 216. ISBN 9780080860497. Retrieved 17 May 2015.
- Gareth Griffiths (1993). Fine Structure Immunocytochemistry. Berlin & Heidelberg: Springer. p. 185. ISBN 978-3-642-77097-5.
- Dar MS; Ikram M (1979). Studies on Quercus infectoria; isolation of syringic acid and determination of its central depressive activity. Planta Med. PMID 419182.
- Dar MS; Ikram M (1979). "Studies on Quercus infectoria; isolation of syringic acid and determination of its central depressive activity". Planta Medica. Planta Med. 35 (2): 156–61. doi:10.1055/s-0028-1097197. PMID 419182.
- Hwang JK; Kong TW; Baek NI; Pyun YR (2000). alpha-Glycosidase inhibitory activity of hexagalloylglucose from the galls of Quercus infectoria. Planta Med.
- Hwang JK; Kong TW; Baek NI; Pyun YR (2000). alpha-Glycosidase inhibitory activity of hexagalloylglucose from the galls of Quercus infectoria. Planta Med.
- Ikram M; Nowshad F (1997). "Constituents of Quercus infectoria". Planta Medica. Planta Med. 31 (3): 286–7. doi:10.1055/s-0028-1097531. PMID 866492.PubMed
- Evans WC (1996). Pharmacopoeial and related drugs of biological origin. London: WB Saunders Co. Ltd.
- Wiart C; Kumar A (2001). Practical Handbook of Pharmacognosy. Malaysia: Pearson Education Malaysia Sdn Bhd.
- Regina Fölster-Holst M.D.; Eva Latussek Ph.D.2 (2007). Synthetic Tannins in Dermatology — A Therapeutic Option in a Variety of Pediatric Dermatoses. Department of Dermatology, Venerology and Allergology, University of Schleswig-Holstein, Kiel.Wiley Online Library
- Hwang JK; Kong TW; Baek NI; Pyun YR (2000). α-Glycosidase Inhibitory Activity of hexagalloylglucose from the galls of Quercus infectoria. Planta Med.PubMed
- Dar MS; Ikram M; Fakouhi T (1976). "Pharmacology of Quercus infectoria". Journal of Pharmaceutical Sciences. J Pharm Sci. 65 (12): 1791–4. doi:10.1002/jps.2600651224. PMID 1032663.PubMedPubMed
- Hussein G; Miyashiro H; Nakamura N; Hattori M; Kakiuchi N; Shimotohno K (2000). Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus protease. Phytother Res.Ful Text
- Fatima S; Farooqi AHA; Kumar R; Kumar TRS; Khanuja SPS (2001). Antibacterial activity possessed by medicinal plants used in tooth powders. J Med Aromatic Plant Sci.
- Digraki M; Alma MH; Ilcim A; Sen S (1999). Antibacterial and antifungal effects of various commercial plant extracts. Pharm Biol.
- Redwane A; Lazrek HB; Bouallam S; Markouk M; Amarouch H; Jana M (2002). Larvicidal activity of extracts from Quercus lusitania var. infectoria galls (Oliv.). J Ethnopharmacol.PubMedFull Text
- Kaur G; Hamid H; Ali A; Alam MS; Athar M (2004). "Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria". Journal of Ethnopharmacology. J Ethnopharmacol. 90 (2–3): 285–92. doi:10.1016/j.jep.2003.10.009. PMID 15013194.PubMedFull Text
- Ikram M; Nowshad F (1997). "Constituents of Quercus infectoria". Planta Medica. Planta Med. 31 (3): 286–7. doi:10.1055/s-0028-1097531. PMID 866492.PubMed
- Evans WC (1996). Pharmacopoeial and related drugs of biological origin. London: WB Saunders Co. Ltd.
- Wiart C; Kumar A (2001). Practical Handbook of Pharmacognosy. Malaysia: Pearson Education Malaysia Sdn Bhd.
- Sawangjaroen; Sawangjaroen K; Poonpanang P.; et al. (2004). "Effects of Piper longum fruit, Piper sarmentosum root and Quercus infectoria nut gall on caecal amoebiasis in mice". Journal of Ethnopharmacology. J Ethnopharmacol. 91 (2–3): 357–60. doi:10.1016/j.jep.2004.01.014. PMID 15120461.PubMed
- Kaur G; Hamid H; Ali A; Alam MS; Athar M (2004). "Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria". Journal of Ethnopharmacology. J Ethnopharmacol. 90 (2–3): 285–92. doi:10.1016/j.jep.2003.10.009. PMID 15013194.PubMed
- Kaur G; Alam MS; Athar M (2007). "Quercus infectoria galls possess antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages". Chemico-Biological Interactions. Chem Biol Interact. 171 (3): 272–82. doi:10.1016/j.cbi.2007.10.002. PMID 18076871.PubMed
- Kaur G; Alam MS; Athar M (2007). "Quercus infectoria galls possess antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages". Chemico-Biological Interactions. Chem Biol Interact. 171 (3): 272–82. doi:10.1016/j.cbi.2007.10.002. PMID 18076871.PubMed
- Kaur G; Hamid H; Ali A; Alam MS; Athar M (2004). "Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria". Journal of Ethnopharmacology. J Ethnopharmacol. 90 (2–3): 285–92. doi:10.1016/j.jep.2003.10.009. PMID 15013194.PubMed
- Dayang F.B.; Hikmah M.I.; Mastura M. (2005). An alternative phytotherapeutic agent for treatment of hospital-acquired MRSA infections. J Med Sci.
- Voravuthikunchai; S.P.; Limsuwan; S. and Chusri (2007). New perspectives on herbal medicines for treating bacterial infections. Houxton: Studium Press.
- Encyclopædia Britannica, Aleppo oak |accessdate=17 May 2015
- Frank Mayer (1988). Electron Microscopy in Microbiology. 20. London: Academic Press. p. 216. ISBN 9780080860497. Retrieved 17 May 2015.
- Gareth Griffiths (1993). Fine Structure Immunocytochemistry. Berlin & Heidelberg: Springer. p. 185. ISBN 978-3-642-77097-5.
- Kottakkal AVS. (1995). Indian Medicinal Plants. 4. Orient Longman Ltd.
- Bhattacharjee SK. (2001). Handbook of Medicinal Plants. India: Pointer Publishers.
- Muhamad Z; Mustafa AM (1994). Traditional Malay Medicinal Plants. Kuala Lumpur: Penerbit Fajar Bakti Sdn Bhd.
External links
- Detailed studies on Quercus infectoria Olivier (nutgalls) as an alternative treatment for methicillin-resistant Staphylococcus aureus infections
- Pharmacognostic studies of insect gall of Quercus infectoria Olivier (Fagaceae).
- The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents
- Gallnuts and the Uses of Tannins in Chinese Medicine
- Detailed studies on Quercus infectoria Olivier (nutgalls) as an alternative treatment for methicillin-resistant Staphylococcus aureus infections
- Pharmacognostic studies of insect gall of Quercus infectoria Olivier (Fagaceae).
- The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents
- Gallnuts and the Uses of Tannins in Chinese Medicine
- Manjakani for vaginal tightening
- Scanning Microscope-Energy Dispersive X-Ray (Sem-Edx) Studies of Quercus infectoria Gall
| Wikimedia Commons has media related to: |