Subtilisin
Subtilisin is a protease (a protein-digesting enzyme) initially obtained from Bacillus subtilis.[2][3][4][5][6][7][8]
| Peptidase S8, subtilisin-related | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Peptidase_S8 | ||||||||
| Pfam | PF00082 | ||||||||
| InterPro | IPR015500 | ||||||||
| PROSITE | PDOC00125 | ||||||||
| CATH | 1cse | ||||||||
| SCOP2 | 1cse / SCOPe / SUPFAM | ||||||||
| CDD | cd07477 | ||||||||
| |||||||||
| Subtilisin BPN' | |||||||
|---|---|---|---|---|---|---|---|
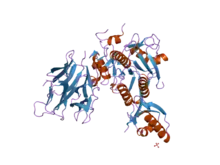
 Crystal structure of subtilisin S8 domain.[1] | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | apr | ||||||
| CAS number | 9014-01-1 | ||||||
| Entrez | 5712479 | ||||||
| PDB | 1st2 More structures | ||||||
| UniProt | P00782 | ||||||
| Other data | |||||||
| EC number | 3.4.21.62 | ||||||
| |||||||
| GO:0004252 | |||||||
Subtilisins belong to subtilases, a group of serine proteases that – like all serine proteases – initiate the nucleophilic attack on the peptide (amide) bond through a serine residue at the active site. Subtilisins typically have molecular weights 27kDa. They can be obtained from certain types of soil bacteria, for example, Bacillus amyloliquefaciens from which they are secreted in large amounts.
Nomenclature
Subtilisin is also commercially known as Alcalase®, Alcalase® 0.6L, Alcalase® 2.5L, ALK-enzyme, bacillopeptidase A, bacillopeptidase B, Bacillus subtilis alkaline proteinase bioprase, bioprase AL 15, bioprase APL 30, colistinase, subtilisin J, subtilisin S41, subtilisin Sendai, subtilisin GX, subtilisin E, subtilisin BL, genenase I, Esperase®, maxatase, thermoase PC 10, protease XXVII, thermoase, superase, subtilisin DY, subtilopeptidase, SP 266, Savinase® 8.0L, Savinase® 4.0T, kazusase, protease VIII, opticlean, Bacillus subtilis alkaline proteinase, protin A 3L, Savinase®, Savinase® 16.0L, Savinase® 32.0 L EX, orientase 10B, protease S). It is the type serine endopeptidase of MEROPS family S8.
Structure
The structure of subtilisin has been determined by X-ray crystallography. The mature form is a 275-residue globular protein with several alpha-helices, and a large beta-sheet. The N-terminal contains an I9 propetide domain (InterPro: IPR010259) that assists the folding of subtilisin. Proteolytic removal of the domain activates the enzyme. It is structurally unrelated to the chymotrypsin-clan of serine proteases, but uses the same type of catalytic triad in the active site. This makes it a classic example of convergent evolution.
Mechanism of catalysis
The active site features a charge-relay network involving Asp-32, His-64, and active site Ser-221 arranged in a catalytic triad. The charge-relay network functions as follows: The carboxylate side-chain of Asp-32 hydrogen-bonds to a nitrogen-bonded proton on His-64's imidazole ring. This is possible because Asp is negatively charged at physiological pH. The other nitrogen on His-64 hydrogen-bonds to the O-H proton of Ser-221. This last interaction results in charge-separation of O-H, with the oxygen atom being more nucleophilic. This allows the oxygen atom of Ser-221 to attack incoming substrates (i.e., peptide bonds), assisted by a neighboring carboxyamide side-chain of Asn-155.
Even though Asp-32, His-64, and Ser-221 are sequentially far apart, they converge in the 3D structure to form the active site.
To summarize the interactions described above, Ser-221 acts as a nucleophile and cleaves peptide bonds with its partially negative oxygen atom. This is possible due to the nature of the charge-relay site of subtilisin.
Applications
Research tool
In molecular biology using B. subtilis as a model organism, the gene encoding subtilisin (aprE) is often the second gene of choice after amyE for integrating reporter constructs into, due to its dispensability.
Commercial
Protein-engineered subtilisins are widely used in commercial products (the native enzyme is easily inactivated by detergents and high temperatures) and is also called a stain cutter, for example, in laundry[9] and dishwashing detergents, cosmetics, food processing,[10] skin care ointments,[11] contact lens cleaners, and for research in synthetic organic chemistry.
Occupational safety and health
People can be exposed to subtilisin in the workplace by breathing it in, swallowing it, skin contact, and eye contact. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.00006 mg/m3 over a 60-minute period.[12]
Subtilisin can cause "enzymatic detergent asthma". People who are sensitive to Subtilisin (Alcalase) usually are also allergic to the bacteria Bacillus subtilis. [13]
References
- PDB: 1st2; Bott R, Ultsch M, Kossiakoff A, Graycar T, Katz B, Power S (June 1988). "The three-dimensional structure of Bacillus amyloliquefaciens subtilisin at 1.8 A and an analysis of the structural consequences of peroxide inactivation". The Journal of Biological Chemistry. 263 (16): 7895–906. PMID 3286644.
- Ottesen M, Svendsen I (1970). "The subtilisins". Methods Enzymol. 19: 199–215. doi:10.1016/0076-6879(70)19014-8.
- Markland FS, Smith EL (1971). "Subtilisins: primary structure, chemical and physical properties". In Boyer PD (ed.). The Enzymes. 3 (3rd ed.). New York: Academic Press. pp. 561–608.
- Philipp M, Bender ML (1983). "Kinetics of subtilisin and thiolsubtilisin". Molecular and Cellular Biochemistry. 51 (1): 5–32. doi:10.1007/bf00215583. PMID 6343835.
- Nedkov P, Oberthür W, Braunitzer G (April 1985). "Determination of the complete amino-acid sequence of subtilisin DY and its comparison with the primary structures of the subtilisins BPN', Carlsberg and amylosacchariticus". Biological Chemistry Hoppe-Seyler. 366 (4): 421–30. doi:10.1515/bchm3.1985.366.1.421. PMID 3927935.
- Ikemura H, Takagi H, Inouye M (June 1987). "Requirement of pro-sequence for the production of active subtilisin E in Escherichia coli". The Journal of Biological Chemistry. 262 (16): 7859–64. PMID 3108260.
- Polgár L (1987). "Structure and function of serine proteases". In Brocklehurst K, Neuberger A (eds.). Hydrolytic enzymes. Amsterdam: Elsevier. ISBN 0-444-80886-8.
- Vasantha N, Thompson LD, Rhodes C, Banner C, Nagle J, Filpula D (September 1984). "Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein". Journal of Bacteriology. 159 (3): 811–9. doi:10.1128/JB.159.3.811-819.1984. PMC 215730. PMID 6090391.
- "Spar Washing Detergent contents".
- Chaplin M (20 December 2004). "Applications of proteases in the food industry". London South Bank University. Archived from the original on 2010-03-14. Retrieved 3 March 2015.
- "Callex® Ointment". Archived from the original on 2008-02-03. Retrieved 3 March 2015.
- "CDC - NIOSH Pocket Guide to Chemical Hazards - Subtilisins". www.cdc.gov. Retrieved 2015-11-21.
- Mosby's Medical, Nursing, & Allied Health Dictionary, 14th edition, page 557
