Taxine alkaloids
Taxine alkaloids, which are often named under the collective title of taxines, are the toxic chemicals that can be isolated from the yew tree.[2][3] The amount of taxine alkaloids depend on the species of yew, with Taxus baccata and Taxus cuspidata containing the most.[4] The major taxine alkaloids are taxine A and taxine B although there are at least 10 different alkaloids.[5] Until 1956, it was believed that all the taxine alkaloids were one single compound named taxine.[4]
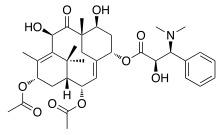 Taxine A | |
| Names | |
|---|---|
| IUPAC name
2α,13α-Diacetoxy-7β,10β-dihydroxy-9-oxo-2(3→20)-abeotaxa-4(20),11-dien-5α-yl (2R,3S)-3-(dimethylamino)-2-hydroxy-3-phenylpropanoate | |
| Other names
(1R,2S,3E,5S,7S,8S,10R,13S)-2,13-Diacetoxy-7,10-dihydroxy-8,12,15,15-tetramethyl-9-oxotricyclo[9.3.1.14,8]hexadeca-3,11-dien-5-yl (2R,3S)-3-(dimethylamino)-2-hydroxy-3-phenylpropanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C35H47NO10 | |
| Molar mass | 641.751 g·mol−1 |
| Melting point | 204-206 °C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
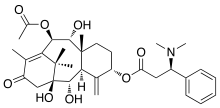 Taxine B | |
| Names | |
|---|---|
| IUPAC name
10β-Acetoxy-1,2α,9α-trihydroxy-13-oxotaxa-4(20),11-dien-5α-yl (3R)-3-(dimethylamino)-3-phenylpropanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C33H45NO8 | |
| Molar mass | 583.722 g·mol−1 |
| Melting point | 115 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The taxine alkaloids are cardiotoxins with taxine B being the most active.[6] Taxine alkaloids have no medical uses but Paclitaxel and other taxanes that can be isolated from yews have been used as chemotherapy drugs.[7]
Provenance

Taxine can be found in Taxus species: Taxus cuspidata, T. baccata (English yew), Taxus x media, Taxus canadensis, Taxus floridana and Taxus brevifolia (Pacific or western yew). All of these species contain taxine in every part of the plant except in the aril, the fleshy covering of the seeds (berries). Each species contains a different amount of taxine concentration, which leads to varying toxicities within the genus. This is the case of the Taxus brevifolia or Pacific yew and the Taxus baccata (English yew); T. baccata contains high taxine concentration, which leads to a high toxicity, whereas T. brevifolia has a low toxicity. There are seasonal changes in the concentrations of taxine in yew plants, with the highest concentrations during the winter, and the lowest in the summer.[8] Taxus species are one of the few trees whose poison is not denatured upon death; in other words, the effect of the Taxine continues functioning independently of the life of the yew in which it is contained.[9]
These species have distinctive leaves, which are needle-like, small, spirally arranged but twisted so they are two-ranked, linear-lanceolate. They are also characterized by their ability to regenerate from stumps and roots.[8] They are found all over Europe and live in conditions similar to the oceanic specia and better in mixed forests (coniferous), on limestone substrates, and occupy rock cliffs and slopes. They are sensitive to low temperatures, the reason why they cannot be found in Northern Scandinavia.[10]
History
The toxic nature of yew trees has been known for millennia.[11] There are a number of early recorded examples of poisonings by Greek and Roman writers including Julius Caesar's account of Cativolcus, king of Eburones, who committed suicide using the “juice of the yew”.[12] The first attempt to extract the poisonous substance in the yew tree was in 1828 by Piero Peretti who isolated a bitter substance.[13] In 1856, H. Lucas, a pharmacist in Arnstadt, prepared a white, alkaloid powder from the foliage of Taxus baccata L. which he named taxine.[14] The crystalline form of the substance was isolated in 1876 by W. Marmé, a French chemist. A. Hilger and F. Brande used elemental combustion analysis in 1890 to suggest the first molecular formula of .[4]
For the next 60 years, it was generally accepted that taxine was made of a single compound and it was well known enough for Agatha Christie to use it as a poison in A Pocket Full of Rye (1953). However, in 1956, Graf and Boeddeker discovered that Taxine was actually a complex mixture of alkaloids rather than a single alkaloid.[15] Using electrophoresis, they were able to isolate the two major components, Taxine A and Taxine B. Taxine A was the fastest moving band and accounted for 1.3% of the alkaloid mixture and Taxine B was the slowest moving band and accounted for 30% of the mixture.[16] The full structure of Taxine A was reported in 1982[1] and of Taxine B in 1991.[17]
Toxicity in humans


The entire yew bush, except the aril (the red flesh of the berry covering the seed), is toxic due to a group of chemicals called Taxine alkaloids. Their cardiotoxicity is well known and act via calcium and sodium channel antagonism, causing an increase in cytoplasmic calcium currents of the myocardial cells. The seeds contains the highest concentrations of these alkaloids.[18][19] If any leaves or seeds of the plant are ingested, urgent medical advice is recommended as well as observation for at least 6 hours after the point of ingestion.[20][21] The most cardiotoxic Taxine is Taxine B followed by Taxine A - Taxine B also happens to be the most common alkaloid in the Taxus species.[6][22][4] The Taxine alkaloids are absorbed quickly from the intestine and in high enough quantities can cause death due to general cardiac failure, cardiac arrest or respiratory failure.[23] Taxines are also absorbed efficiently via the skin and Taxus species should thus be handled with care and preferably with gloves.[24] Taxus Baccata leaves contain approximately 5 mg of Taxines per 1g of leaves.[22] The estimated lethal dose (LDmin) of Taxus baccata leaves is 3.0-6.5 mg/kg body weight for humans[25] There is currently no known antidotes for yew poisoning, but drugs such as atropine have been used to treat the symptoms.[26] Taxine remains in the plant all year, with maximal concentrations appearing during the winter. Dried yew plant material retains its toxicity for several months and even increases its toxicity as the water is removed,[27] fallen leaves are also toxic.[28] Although poisoning usually occurs when leaves of yew trees are eaten, in at least one case a victim inhaled sawdust from a yew tree.[29] One major reason as to why different studies show different LD results is that it is difficult to measure Taxine alkaloids accurately.[30]
Male and monoecious yews in this genus release toxic pollen, which can cause the mild symptoms of Taxine poisoning. The pollen are also a trigger for asthma. These pollen grains are only 15 microns in size, and can easily pass through most window screens.[31]
Clinical signs
Cardiac and cardiovascular effects:
Arrhythmia, irregular heartbeats leading to lower cardiac output - in itself is a very severe symptom, especially ventricular arrytmias can lead to circulatory collapse(via cardiac arrest) very fast if not treated
Bradycardia, fewer heart beats per time unit.
Both these effects lead to:
Hypotension which gives many symptoms such as:
and many other typical signs of low blood pressure
Intestinal effects:
- Nausea and vomiting
- Diarrhoea
- Abdominal pain
Respiratory effects:
- Respiratory distress(shortness of breath)
If the poisoning is severe and not treated:
- Loss of consciousness - lack of oxygen due to low blood pressure and respiratory distress forces the body to shut down all but the most vital functions.
- Respiratory failure, breathing stops
- Circulatory collapse, the blood pressure drops to the point that not even the most basic functions can be sustained
Diagnosis
The diagnosis of yew poisoning is very important if the patient is not already aware of having ingested parts of the yew tree. The method of diagnosis is the determination of 3,5-dimethoxyphenol, a product of the hydrolysis of the glycosidic bond in taxine, in the blood, the gastric contents, the urine and the tissues of the patient. This analysis can be done by gas or liquid chromatography and also by mass spectroscopy.
Treatment
There are no specific antidotes for taxine, so patients can only receive treatment for their symptoms.
It is also important to control the blood pressure and the heart rate to treat the heart problems. Atropine has been used successfully in humans to treat bradycardias and arrhythmias caused by taxine. It is more effective if administrated early, but it is also necessary to be cautious with the administration because it can produce an increase in myocardial oxygen demand and potentiate myocardial hypoxia and dysfunction. An artificial cardiac pacemaker can also be installed to control the heartbeat.
Other treatments are useful to treat the other symptoms of poisoning: positive pressure ventilation if respiratory distress is present; fluid therapy to support blood pressure and maintain hydration and renal function; gastrointestinal protectants; and it may also be necessary to control aggressive behaviour and convulsions with tranquilizers.[35]
Prevention
The toxic effects of T. baccata have been known since ancient times. In most cases, poisoning is accidental, especially in cases involving children or animals. However, there are cases in which the poison is used as a suicide method.[36]
Because taxine poisoning is often only diagnosed after the death of the patient due to its rapid effect, preventing exposure is very important. Even dried parts of the plant are toxic because they still contain taxine molecules. Pet owners must ensure that yew branches or leaves are not used as toys for dogs or as perches for domestic birds.
Toxicity in animals
The effects of Taxine in humans are very similar to the effects on animals. It has the same mechanisms of action, and most of the times the ingestion of yew material is diagnosed with the death of the animal. Moreover, clinical signs, diagnosis, treatment and prevention are mostly the same as in humans. This was seen due to the many experiments realized on rats, pigs, and other animals.[8]
However, there are some animals that are immune to the effects of taxine such macaws and white-tail deer.
Mechanism of action
The toxicity of the yew plant is due to some substances that are found in it, the principal ones are: toxic alkaloids (taxine B, paclitaxel, isotaxine B, taxine A), glycosides (taxicatine) and taxane derivates (taxol A, taxol B).[37]
There have been many studies about the toxicity of the taxine alkaloids[38][39] and they have shown that their mechanism of action is interfering with the sodium and calcium channels of the myocardial cells, increasing the cytoplasmic calcium concentrations. Their mechanism is similar to drugs such as verapamil, although taxines are more cardioselective.[40] They also reduce the rate of the depolarization of the action potential in a dose-dependent manner. This produces bradycardia, hypotension, depressed myocardial contractility, conduction delay, arrhythmias, and other complications.[41]
Some taxine alkaloids have been isolated to study its effects and characteristics, this has allowed the discovery of some of the particular effects of each substance of the plant, for example: taxine A does not influence blood pressure, taxol causes cardiac disturbances in some people, and that taxine B is the most toxic of these substances.[42]
Because a derivative from the yew, paclitaxel, functions as an anticancer drug, there have been investigations to show whether taxine B could also be used as a pharmacodrug.[43]
See also
References
- Graf, Engelbert; Kirfel, Armin; Wolff, Gerd-Joachim; Breitmaier, Eberhard (1982-02-10). "Die Aufklärung von Taxin A ausTaxus baccata L.". Liebigs Annalen der Chemie. 1982 (2): 376–381. doi:10.1002/jlac.198219820222. ISSN 0170-2041.
- Veterinary toxicology : basic and clinical principles. Gupta, Ramesh C. (Ramesh Chandra), 1949- (2nd ed.). Oxford: Academic. 2012. ISBN 9780123859266. OCLC 778786624.CS1 maint: others (link)
- Miller, Roger W. (July 1980). "A Brief Survey of Taxus Alkaloids and Other Taxane Derivatives". Journal of Natural Products. 43 (4): 425–437. doi:10.1021/np50010a001. ISSN 0163-3864.
- Wilson, Christina R.; Sauer, John-Michael; Hooser, Stephen B. (2001). "Taxines: a review of the mechanism and toxicity of yew (Taxus spp.) alkaloids". Toxicon. 39 (2–3): 175–185. doi:10.1016/s0041-0101(00)00146-x. ISSN 0041-0101. PMID 10978734.
- Constable, Peter D (2016-10-25). Veterinary medicine : a textbook of the diseases of cattle, horses, sheep, pigs and goats. Hinchcliff, Kenneth W. (Kenneth William), 1956-, Done, Stanley H.,, Grünberg, Walter,, Preceded by: Radostits, O. M. (11th ed.). St. Louis, Mo. ISBN 9780702070587. OCLC 962414947.
- Alloatti, G.; Penna, C.; Levi, R. C.; Gallo, M. P.; Appendino, G.; Fenoglio, I. (1996). "Effects of yew alkaloids and related compounds on guinea-pig isolated perfused heart and papillary muscle". Life Sciences. 58 (10): 845–854. doi:10.1016/0024-3205(96)00018-5. ISSN 0024-3205. PMID 8602118.
- Muriel, Le Roux (2016-11-26). Navelbine® and Taxotère® : histories of sciences. Gueritte, Françoise. London. ISBN 9780081011379. OCLC 964620092.
- http://aspcapro.org/sites/pro/files/zk_vetm0905_646_650.pdf
- "Taxus baccata, yew - THE POISON GARDEN website".
- Farjon, A. (2013). "Taxus baccata (errata version published in 2017)". IUCN Red List of Threatened Species. 2013: e.T42546A117052436. doi:10.2305/IUCN.UK.2013-1.RLTS.T42546A2986660.en. Retrieved 15 January 2020.{{cite iucn}}: error: |doi= / |page= mismatch (help)
- F.), Roberts, M. F. (Margaret (1998). Alkaloids : Biochemistry, Ecology, and Medicinal Applications. Wink, Michael. Boston, MA: Springer US. ISBN 9781475729054. OCLC 851770197.
- Julius., Caesar (1982). "31". De bello Gallico VI. Kennedy, E. C. (Eberhard Christopher). Bristol: Bristol Classical Press, Dept. of Classics, University of Bristol. ISBN 978-0862920883. OCLC 12217646.
- Appendino, Giovanni (1996). Alkaloids: Chemical and Biological Perspectives. 11. Elsevier. pp. 237–268. doi:10.1016/s0735-8210(96)80006-9. ISBN 9780080427973.
- Lucas, H. (1856). "Ueber ein in den Blättern von Taxus baccata L. enthaltenes Alkaloid (das Taxin)". Archiv der Pharmazie. 135 (2): 145–149. doi:10.1002/ardp.18561350203. ISSN 0365-6233. S2CID 84660890.
- Graf, E (1956-04-07). "Zur chemie des taxins". Angewandte Chemie (in German). 68 (7): 249–250. doi:10.1002/ange.19560680709. ISSN 0044-8249.
- Wilson, Christina R.; Hooser, Stephen B. (2007). Veterinary Toxicology. Elsevier. pp. 929–935. doi:10.1016/b978-012370467-2/50171-1. ISBN 9780123704672.
- Ettouati, L.; Ahond, A.; Poupat, C.; Potier, P. (September 1991). "Révision Structurale de la Taxine B, Alcaloïde Majoritaire des Feuilles de l'If d'Europe, Taxus baccata". Journal of Natural Products. 54 (5): 1455–1458. doi:10.1021/np50077a044. ISSN 0163-3864.
- Garland, Tam; Barr, A. Catherine (1998). Toxic plants and other natural toxicants. International Symposium on Poisonous Plants (5th : 1997 : Texas). Wallingford, England: CAB International. ISBN 0851992633. OCLC 39013798.
- Alloatti, G.; Penna, C.; Levi, R.C.; Gallo, M.P.; Appendino, G.; Fenoglio, I. (1996). "Effects of yew alkaloids and related compounds on guinea-pig isolated perfused heart and papillary muscle". Life Sciences. 58 (10): 845–854. doi:10.1016/0024-3205(96)00018-5. ISSN 0024-3205. PMID 8602118.
- "TOXBASE - National Poisons Information Service".
- "Taxus baccata - L." Plants for a Future. Retrieved 2019-07-17.
- Smythies, J.R.; Benington, F.; Morin, R.D.; Al-Zahid, G.; Schoepfle, G. (1975). "The action of the alkaloids from yew (Taxus baccata) on the action potential in the Xenopus medullated axon". Experientia. 31 (3): 337–338. doi:10.1007/bf01922572. PMID 1116544. S2CID 8927297.
- Fuller, Thomas C.; McClintock, Elizabeth M. (1986). Poisonous plants of California. Berkeley: University of California Press. ISBN 0520055683. OCLC 13009854.
- Mitchell, A. F. (1972). Conifers in the British Isles. Forestry Commission Booklet 33.
- Watt, J.M.; Breyer-Brandwijk, M.G. (1962). Taxaceae. In The Medicinal and Poisonous Plants of Southern and Eastern Africa. Edinburgh, UK: Livingston. pp. 1019–1022.
- Wilson, Christina R.; Hooser, Stephen B. (2018). Veterinary Toxicology. Elsevier. pp. 947–954. doi:10.1016/b978-0-12-811410-0.00066-0. ISBN 9780128114100.
- "Yew". Provet. Retrieved 23 March 2013.
- "Intoxicación por tejo". fetoc.es.
- José Luis Hernández Hernández; Fernando Quijano Terán; Jesús González Macías (2010). "Intoxicación por tejo". Medicina Clínica. 135 (12): 575–576. doi:10.1016/j.medcli.2009.06.036. PMID 19819481.
- Clarke, E.G.C.; Clarke, M.L. (1988). Veterinary Toxicology:Poisonous plants, Taxaceae (3rd ed.). London: Baillière, Tindall & Cassell. pp. 276–277.
- "Pollen images in size order". www.saps.org.uk. Retrieved 2017-04-13.
- "Taxine". Toxnet.
- "Yew poisoning". MedlinePlus.
- "PLANT POISONING, CERVID - USA: (ALASKA) ORNAMENTAL TREE, MOOSE". ProMED-mail. 22 February 2011. Retrieved 25 January 2016.
- R.B. Cope (September 2005). "The dangers of yew ingestion" (PDF). Veterinary Medicine.
- "Suicidal poisoning by ingestion of Taxus Baccata leaves. Case report and literature review" (PDF). Romanian Journal of Legal Medicine. XXI (2(2013)).
- Ramachandran, Dr. Sundaram (2014). Heart and Toxins. p. 160. ISBN 9780124165953.
- "Taxine- Mechanism of action". PubChem.
- Tekol Y (1985). "Negative chronotropic and atrioventricular blocking effects of taxine on isolated frog heart and its acute toxicity in mice". Planta Medica. 51 (5): 357–60. doi:10.1055/s-2007-969519. PMID 17342582.
- Tekol Y; Göğüsten B (1999). "Comparative determination of the cardioselectivity of taxine and verapamil in the isolated aorta, atrium and jejunum preparations of rabbits". Arzneimittelforschung. 49 (8): 673–8. doi:10.1055/s-0031-1300481. PMID 10483513.
- G. Barceloux, Donald (2008). Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals. pp. 900. ISBN 9780471727613.
- Suffness, Matthew (1995). Taxol: Science and Applications. p. 311.
- Barbara Andersen, Karina (2009). "Future perspectives of the role of Taxines derived from the Yew (Taxus baccata) in research and therapy". Journal of Pre-Clinical and Clinical Research. 3 (1).
Further reading
Asheesh K. Tiwary, Birgit Puschner, Hailu Kinde, Elizabeth R. Tor (2005). "Diagnosis of Taxus (Yew) poisoning in a horse". Journal of Veterinary Diagnostic Investigation.
Andrea Persico, Giuseppe Bacis, Francesca Uberti, Claudia Panzeri, Chiara Di Lorenzo, Enzo Moro, and Patrizia Restani (2011). "Identification of Taxine Derivatives in Biological Fluids from a Patient after Attempted Suicide by Ingestion of Yew (Taxus baccata) Leaves". Journal of Analytical Toxicology. Vol. 35