Artificial cardiac pacemaker
A cardiac pacemaker (or artificial pacemaker, so as not to be confused with the natural pacemaker of the heart), is a medical device that generates electrical impulses delivered by electrodes to cause the heart muscle chambers (the upper, or atria and/or the lower, or ventricles) to contract and therefore pump blood; by doing so this device replaces and/or regulates the function of the electrical conduction system of the heart.
| Artificial cardiac pacemaker | |
|---|---|
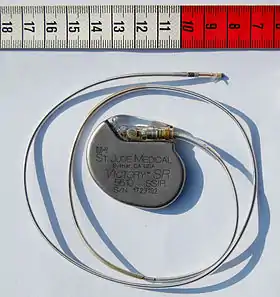 St Jude Medical pacemaker with ruler |
The primary purpose of a pacemaker is to maintain an adequate heart rate, either because the heart's natural pacemaker is not fast enough, or because there is a block in the heart's electrical conduction system. Modern pacemakers are externally programmable and allow a cardiologist, particularly a cardiac electrophysiologist to select the optimal pacing modes for individual patients. A specific type of pacemaker called a defibrillator combines pacemaker and defibrillator functions in a single implantable device, which should be called a defibrillator, for clarity. Others, called biventricular pacemakers have multiple electrodes stimulating differing positions within the lower heart chambers to improve synchronization of the ventricles, the lower chambers of the heart.
Methods of pacing
Percussive pacing
Percussive pacing, also known as transthoracic mechanical pacing, is the use of the closed fist, usually on the left lower edge of the sternum over the right ventricle in the vena cava, striking from a distance of 20 – 30 cm to induce a ventricular beat (the British Journal of Anaesthesia suggests this must be done to raise the ventricular pressure to 10–15 mmHg to induce electrical activity). This is an old procedure used only as a life saving means until an electrical pacemaker is brought to the patient.[1]
Transcutaneous pacing
Transcutaneous pacing (TCP), also called external pacing, is recommended for the initial stabilization of hemodynamically significant bradycardias of all types. The procedure is performed by placing two pacing pads on the patient's chest, either in the anterior/lateral position or the anterior/posterior position. The rescuer selects the pacing rate, and gradually increases the pacing current (measured in mA) until electrical capture (characterized by a wide QRS complex with a tall, broad T wave on the ECG) is achieved, with a corresponding pulse. Pacing artifact on the ECG and severe muscle twitching may make this determination difficult. External pacing should not be relied upon for an extended period of time. It is an emergency procedure that acts as a bridge until transvenous pacing or other therapies can be applied.
Epicardial pacing (temporary)

Temporary epicardial pacing is used during open heart surgery should the surgical procedure create atrio-ventricular block. The electrodes are placed in contact with the outer wall of the ventricle (epicardium) to maintain satisfactory cardiac output until a temporary transvenous electrode has been inserted.
Transvenous pacing (temporary)
Transvenous pacing, when used for temporary pacing, is an alternative to transcutaneous pacing. A pacemaker wire is placed into a vein, under sterile conditions, and then passed into either the right atrium or right ventricle. The pacing wire is then connected to an external pacemaker outside the body. Transvenous pacing is often used as a bridge to permanent pacemaker placement. It can be kept in place until a permanent pacemaker is implanted or until there is no longer a need for a pacemaker and then it is removed.
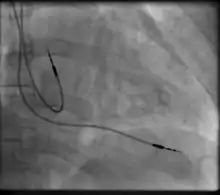
Permanent transvenous pacing
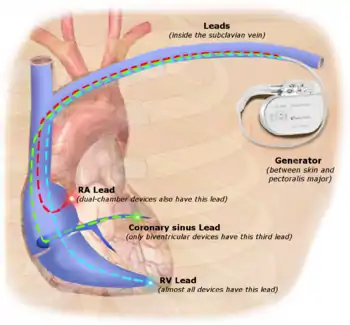
Permanent pacing with an implantable pacemaker involves transvenous placement of one or more pacing electrodes within a chamber, or chambers, of the heart, while the pacemaker is implanted inside the skin under the clavicle. The procedure is performed by incision of a suitable vein into which the electrode lead is inserted and passed along the vein, through the valve of the heart, until positioned in the chamber. The procedure is facilitated by fluoroscopy which enables the physician to view the passage of the electrode lead. After satisfactory lodgement of the electrode is confirmed, the opposite end of the electrode lead is connected to the pacemaker generator.
There are three basic types of permanent pacemakers, classified according to the number of chambers involved and their basic operating mechanism:[2]
- Single-chamber pacemaker. In this type, only one pacing lead is placed into a chamber of the heart, either the atrium or the ventricle.[2]
- Dual-chamber pacemaker. Here, wires are placed in two chambers of the heart. One lead paces the atrium and one paces the ventricle. This type more closely resembles the natural pacing of the heart by assisting the heart in coordinating the function between the atria and ventricles.[2]
- Biventricular pacemaker. This pacemaker has three wires placed in three chambers of the heart. One in the atrium and two in either ventricle. It is more complicated to implant.[2]
- Rate-responsive pacemaker. This pacemaker has sensors that detect changes in the patient's physical activity and automatically adjust the pacing rate to fulfill the body's metabolic needs.[2]
The pacemaker generator is a hermetically sealed device containing a power source, usually a lithium battery, a sensing amplifier which processes the electrical manifestation of naturally occurring heart beats as sensed by the heart electrodes, the computer logic for the pacemaker and the output circuitry which delivers the pacing impulse to the electrodes.
Most commonly, the generator is placed below the subcutaneous fat of the chest wall, above the muscles and bones of the chest. However, the placement may vary on a case by case basis.
The outer casing of pacemakers is so designed that it will rarely be rejected by the body's immune system. It is usually made of titanium, which is inert in the body.
Leadless pacing
Leadless pacemakers are devices that are small enough to allow the generator to be placed within the heart, therefore avoiding the need for pacing leads.[3] As pacemaker leads can fail over time, a pacing system that avoids these components offers theoretical advantages. Leadless pacemakers can be implanted into the heart using a steerable catheter fed into the femoral vein via an incision in the groin.[3]
Basic function

.jpg.webp)
Modern pacemakers usually have multiple functions. The most basic form monitors the heart's native electrical rhythm. When the pacemaker wire or "lead" does not detect heart electrical activity in the chamber - atrium or ventricle - within a normal beat-to-beat time period - most commonly one second - it will stimulate either the atrium or the ventricle with a short low voltage pulse. If it does sense electrical activity, it will hold off stimulating. This sensing and stimulating activity continues on a beat by beat basis and is called "demand pacing". In the case of a dual chamber device, when the upper chambers have a spontaneous or stimulated activation, the device starts a countdown to ensure that in an acceptable - and programmable - interval, there is an activation of the ventricle, otherwise again an impulse will be delivered.
The more complex forms include the ability to sense and/or stimulate both the atrial and ventricular chambers.
| I | II | III | IV | V |
|---|---|---|---|---|
| Chamber(s) paced | Chamber(s) sensed | Response to sensing | Rate modulation | Multisite pacing |
| O = None | O = None | O = None | O = None | O = None |
| A = Atrium | A = Atrium | T = Triggered | R = Rate modulation | A = Atrium |
| V = Ventricle | V = Ventricle | I = Inhibited | V = Ventricle | |
| D = Dual (A+V) | D = Dual (A+V) | D = Dual (T+I) | D = Dual (A+V) |
From this the basic ventricular "on demand" pacing mode is VVI or with automatic rate adjustment for exercise VVIR – this mode is suitable when no synchronization with the atrial beat is required, as in atrial fibrillation. The equivalent atrial pacing mode is AAI or AAIR which is the mode of choice when atrioventricular conduction is intact but the natural pacemaker the sinoatrial node is unreliable – sinus node disease (SND) or sick sinus syndrome. Where the problem is atrioventricular block (AVB) the pacemaker is required to detect (sense) the atrial beat and after a normal delay (0.1–0.2 seconds) trigger a ventricular beat, unless it has already happened – this is VDD mode and can be achieved with a single pacing lead with electrodes in the right atrium (to sense) and ventricle (to sense and pace). These modes AAIR and VDD are unusual in the US but widely used in Latin America and Europe.[5][6] The DDDR mode is most commonly used as it covers all the options though the pacemakers require separate atrial and ventricular leads and are more complex, requiring careful programming of their functions for optimal results.
Biventricular pacing
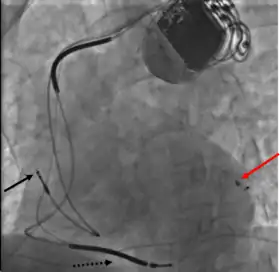
Cardiac resynchronization therapy (CRT) is used for people with heart failure in whom the left and right ventricles do not contract simultaneously (ventricular dyssynchrony), which occurs in approximately 25–50% of heart failure patients. To achieve CRT, a biventricular pacemaker (BVP) is used, which can pace both the septal and lateral walls of the left ventricle. By pacing both sides of the left ventricle, the pacemaker can resynchronize the ventricular contractions.
CRT devices have at least two leads, one passing through the vena cava and the right atrium into the right ventricle to stimulate the septum, and another passing through the vena cava and the right atrium and inserted through the coronary sinus to pace the epicardial wall of the left ventricle. Often, for patients in normal sinus rhythm, there is also a lead in the right atrium to facilitate synchrony with the atrial contraction. Thus, timing between the atrial and ventricular contractions, as well as between the septal and lateral walls of the left ventricle can be adjusted to achieve optimal cardiac function.
CRT devices have been shown to reduce mortality and improve quality of life in patients with heart failure symptoms; a LV ejection fraction less than or equal to 35% and QRS duration on EKG of 120 ms or greater.[7][8]
Biventricular pacing alone is referred to as CRT-P (for pacing). For selected patients at risk of arrhythmias, CRT can be combined with an implantable cardioverter-defibrillator (ICD): such devices, known as CRT-D (for defibrillation), also provide effective protection against life-threatening arrhythmias.[9]
His bundle pacing
Conventional placement of ventricular leads in or around the tip or apex of the right ventricle, or RV apical pacing, can have negative effects on heart function. Indeed, it has been associated with increased risk of atrial fibrillation, heart failure, weakening of the heart muscle and potentially shorter life expectancy. His bundle pacing (HBP) leads to a more natural or perfectly natural ventricular activation and has generated strong research and clinical interest. By stimulating the His–Purkinje fiber network directly with a special lead and placement technique, HBP causes a synchronized and therefore more effective ventricular activation and avoid long term heart muscle disease. HBP in some cases can also correct bundle branch block patterns.[10][11]
Advancements in function

A major step forward in pacemaker function has been to attempt to mimic nature by utilizing various inputs to produce a rate-responsive pacemaker using parameters such as the QT interval, pO2 – pCO2 (dissolved oxygen or carbon dioxide levels) in the arterial-venous system, physical activity as determined by an accelerometer, body temperature, ATP levels, adrenaline, etc. Instead of producing a static, predetermined heart rate, or intermittent control, such a pacemaker, a 'Dynamic Pacemaker', could compensate for both actual respiratory loading and potentially anticipated respiratory loading. The first dynamic pacemaker was invented by Anthony Rickards of the National Heart Hospital, London, UK, in 1982.[12]
Dynamic pacemaking technology could also be applied to future artificial hearts. Advances in transitional tissue welding would support this and other artificial organ/joint/tissue replacement efforts. Stem cells may be of interest in transitional tissue welding.
Many advancements have been made to improve the control of the pacemaker once implanted. Many of these have been made possible by the transition to microprocessor controlled pacemakers. Pacemakers that control not only the ventricles but the atria as well have become common. Pacemakers that control both the atria and ventricles are called dual-chamber pacemakers. Although these dual-chamber models are usually more expensive, timing the contractions of the atria to precede that of the ventricles improves the pumping efficiency of the heart and can be useful in congestive heart failure.
Rate responsive pacing allows the device to sense the physical activity of the patient and respond appropriately by increasing or decreasing the base pacing rate via rate response algorithms.
The DAVID trials[13] have shown that unnecessary pacing of the right ventricle can exacerbate heart failure and increases the incidence of atrial fibrillation. The newer dual chamber devices can keep the amount of right ventricle pacing to a minimum and thus prevent worsening of the heart disease.
Considerations
Insertion
A pacemaker may be implanted whilst a person is awake using local anesthetic to numb the skin with or without sedation, or asleep using a general anesthetic.[14] An antibiotic is usually given to reduce the risk of infection.[14] Pacemakers are generally implanted in the front of the chest in the region of the left or right shoulder. The skin is prepared by clipping or shaving any hair over the implant site before cleaning the skin with a disinfectant such as chlorhexidine. An incision is made below the collar bone and a space or pocket is created under the skin to house the pacemaker generator. This pocket is usually created just above the pectoralis major muscle (prepectoral), but in some cases the device may be inserted beneath the muscle (submuscular).[15] The lead or leads are fed into the heart through a large vein guided by X-ray imaging (fluoroscopy). The tips of the leads may be positioned within the right ventricle, the right atrium, or the coronary sinus, depending on the type of pacemaker required.[14] Surgery is typically completed within 30 to 90 minutes. Following implantation, the surgical wound should be kept clean and dry until it has healed. Care should be taken to avoid excessive movement of the shoulder within the first few weeks to reduce the risk of dislodging the pacemaker leads.[14]
The batteries within a pacemaker generator typically last 5 to 10 years. When the batteries are nearing the end of life, the generator is replaced in a procedure that is usually simpler than a new implant. Replacement involves making an incision to remove the existing device, disconnecting the leads from the old device and reconnecting them to a new generator, reinserting the new device and closing the skin.[14]
Periodic pacemaker checkups

Once the pacemaker is implanted, it is periodically checked to ensure the device is operational and performing appropriately. Depending on the frequency set by the following physician, the device can be checked as often as is necessary. Routine pacemaker checks are typically done in-office every six (6) months, though will vary depending upon patient/device status and remote monitoring availability. Newer pacemaker models can also be interrogated remotely, with the patient transmitting their pacemaker data using an at-home transmitter connected to their geographical cellular network. This data can then be accessed by the technician through the device manufacturer's web portal.
At the time of in-office follow-up, the device will be interrogated to perform diagnostic testing. These tests include:
- Sensing: the ability of the device to "see" intrinsic cardiac activity (Atrial and ventricular depolarization).
- Impedance: A test to measure lead integrity. Large and/or sudden increases in impedance can be indicative of a lead fracture while large and/or sudden decreases in impedance can signify a breach in lead insulation.
- Threshold amplitude: The minimum amount of energy (generally in hundredths of volts) required in order to pace the atrium or ventricle connected to the lead.
- Threshold duration: The amount of time that the device requires at the preset amplitude to reliably pace the atrium or ventricle connected to the lead.
- Percentage of pacing: Defines how dependent the patient is on the device, the percentage of time that the pacemaker has been actively pacing since the previous device interrogation.
- Estimated battery life at current rate: As modern pacemakers are "on-demand", meaning that they only pace when necessary, device longevity is affected by how much it is utilized. Other factors affecting device longevity include programmed output and algorithms (features) causing a higher level of current drain from the battery.
- Any events that were stored since the last follow-up, in particular arrhythmias such as atrial fibrillation. These are typically stored based on specific criteria set by the physician and specific to the patient. Some devices have the availability to display intracardiac electrograms of the onset of the event as well as the event itself. This is especially helpful in diagnosing the cause or origin of the event and making any necessary programming changes.
Magnetic fields, MRIs, and other lifestyle issues
A patient's lifestyle is usually not modified to any great degree after insertion of a pacemaker. There are a few activities that are unwise such as full contact sports and activities that involve intense magnetic fields.
The pacemaker patient may find that some types of everyday actions need to be modified. For instance, the shoulder harness of a vehicle seatbelt may be uncomfortable if the harness should fall across the pacemaker insertion site.
If the patient does wish to practice any type of sport or physical activity, special pacemaker protection can be worn to prevent possible physical injuries or damage to the pacemaker leads.
Any kind of an activity that involves intense electro-magnetic fields should be avoided. This includes activities such as arc welding possibly, with certain types of equipment,[16] or maintaining heavy equipment that may generate intense magnetic fields (such as a magnetic resonance imaging (MRI) machine).
However, in February 2011 the FDA approved a new pacemaker device from Medtronic called the Revo MRI SureScan[17] which was the first to be labeled as conditional[18] for MRI use.[19] There are several limitations to its use including certain patients' qualifications and scan settings. An MRI conditional device has to be reprogrammed right before and right after MRI scanning. All the 5 most common cardiac pacing device manufacturers (covering more than 99% of the US market) now have FDA-approved MR-conditional pacemakers.[20]
A 2008 US study has found[21] that the magnetic field created by some headphones included with portable music players or cell phones, when placed within inches of pacemakers, may cause interference.
In addition, according to the American Heart Association, some home devices have a remote potential to cause interference by occasionally inhibiting a single beat. Cellphones available in the United States (less than 3 watts) do not seem to damage pulse generators or affect how the pacemaker works.[22]
Having a pacemaker does not imply that a patient requires the use of antibiotics to be administered before procedures such as dental work.[23] The patient should inform all medical personnel that he or she has a pacemaker. The use of MRI may be ruled out by the patient having a pacemaker manufactured before MRI conditional devices became common, or by the patient having old pacing wires abandoned inside the heart, no longer connected to their pacemaker.
Turning off the pacemaker
A panel of The Heart Rhythm Society, a specialist organization based in Washington, DC found that it was legal and ethical to honor requests by patients, or by those with legal authority to make decisions for patients, to deactivate implanted cardiac devices. Lawyers say that the legal situation is similar to removing a feeding tube, though there is currently no legal precedent involving pacemakers in the United States of America. A patient in the United States is thought to have a right to refuse or discontinue treatment, including a pacemaker that keeps him or her alive. Physicians have a right to refuse to turn it off, but are advised by the HRS panel that they should refer the patient to a physician who will.[24] Some patients believe that hopeless, debilitating conditions, like those brought on by severe strokes or late-stage dementia, can cause so much suffering that they would prefer not to prolong their lives with supportive measures, such as cardiac devices.[25]
Privacy and security
Security and privacy concerns have been raised with pacemakers that allow wireless communication. Unauthorized third parties may be able to read patient records contained in the pacemaker, or reprogram the devices, as has been demonstrated by a team of researchers.[26] The demonstration worked at short range; they did not attempt to develop a long range antenna. The proof of concept exploit helps demonstrate the need for better security and patient alerting measures in remotely accessible medical implants.[26] In response to this threat, Purdue University and Princeton University researchers have developed a prototype firewall device, called MedMon, which is designed to protect wireless medical devices such as pacemakers and insulin pumps from attackers.[27]
Complications
Complications from having surgery to implant a pacemaker are uncommon (each 1-3 % approximately), but could include: infection where the pacemaker is implanted or in the bloodstream; allergic reaction to the dye or anesthesia used during the procedure; swelling, bruising or bleeding at the generator site, or around the heart, especially if the patient is taking blood thinners, elderly, of thin frame or otherwise on chronic steroids use. [29]
A possible complication of dual-chamber artificial pacemakers is 'pacemaker-mediated tachycardia' (PMT), a form of reentrant tachycardia. In PMT, the artificial pacemaker forms the anterograde (atrium to ventricle) limb of the circuit and the atrioventricular (AV) node forms the retrograde limb (ventricle to atrium) of the circuit.[30] Treatment of PMT typically involves reprogramming the pacemaker.[30]
Another possible complication is "pacemaker-tracked tachycardia," where a supraventricular tachycardia such as atrial fibrillation or atrial flutter is tracked by the pacemaker and produces beats from a ventricular lead. This is becoming exceedingly rare as newer devices are often programmed to recognize supraventricular tachycardias and switch to non-tracking modes.
Sometimes the leads, which are small diameter wires, from the pacemaker to the implantation site in the heart muscle will need to be removed. The most common reason for lead removal is infection, however over time leads can degrade due to a number of reasons such as lead flexing.[31] Changes to programming of the pacemaker may overcome lead degradation to some extent. However, a patient who has several pacemaker replacements over a decade or two in which the leads were reused may require a lead replacement surgery.
Lead replacement may be done in one of two ways. Insert a new set of leads without removing the current leads (not recommended as it provides additional obstruction to blood flow and heart valve function) or remove the current leads and then insert replacements. The lead removal technique will vary depending on the surgeon's estimation of the probability that simple traction will suffice to more complex procedures. Leads can normally be disconnected from the pacemaker easily which is why device replacement usually entails simple surgery to access the device and replace it by simply unhooking the leads from the device to replace and hooking the leads to the new device. The possible complications, such as perforation of the heart wall, come from removing the lead{s} from the patient's body.
The other end of a pacemaker lead is actually implanted into the heart muscle with a miniature screw or anchored with small plastic hooks called tines. In addition, the longer the leads have been implanted starting from a year or two, the more likely that they will have attachments to the patient's body at various places in the pathway from device to heart muscle, since the human body tends to incorporate foreign devices into tissue. In some cases, for a lead that has been inserted for a short amount of time, removal may involve simple traction to pull the lead from the body. Removal in other cases is typically done with a laser or cutting device which threads like a cannula with a cutting edge over the lead and is moved down the lead to remove any organic attachments with tiny cutting lasers or similar device.
Pacemaker lead malposition in various locations has been described in the literature. Depending on the location of the pacer lead and symptoms treatment varies.[32]
Another possible complication called twiddler's syndrome occurs when a patient manipulates the pacemaker and causes the leads to be removed from their intended location and causes possible stimulation of other nerves.
Other devices
Sometimes devices resembling pacemakers, called implantable cardioverter-defibrillators (ICDs) are implanted. These devices are often used in the treatment of patients at risk from sudden cardiac death. An ICD has the ability to treat many types of heart rhythm disturbances by means of pacing, cardioversion, or defibrillation. Some ICD devices can distinguish between ventricular fibrillation and ventricular tachycardia (VT), and may try to pace the heart faster than its intrinsic rate in the case of VT, to try to break the tachycardia before it progresses to ventricular fibrillation. This is known as fast-pacing, overdrive pacing, or anti-tachycardia pacing (ATP). ATP is only effective if the underlying rhythm is ventricular tachycardia, and is never effective if the rhythm is ventricular fibrillation.
| I | II | III | IV |
|---|---|---|---|
| Shock chamber | Antitachycardia pacing chamber | Tachycardia detection | Antibradycardia pacing chamber |
| O = None | O = None | E = Electrogram | O = None |
| A = Atrium | A = Atrium | H = Hemodynamic | A = Atrium |
| V = Ventricle | V = Ventricle | V = Ventricle | |
| D = Dual (A+V) | D = Dual (A+V) | D = Dual (A+V) |
| ICD-S | ICD with shock capability only |
| ICD-B | ICD with bradycardia pacing as well as shock |
| ICD-T | ICD with tachycardia (and bradycardia) pacing as well as shock |
History

Origin
In 1889, John Alexander MacWilliam reported in the British Medical Journal (BMJ) of his experiments in which application of an electrical impulse to the human heart in asystole caused a ventricular contraction and that a heart rhythm of 60–70 beats per minute could be evoked by impulses applied at spacings equal to 60–70/minute.[34]
In 1926, Mark C Lidwill of the Royal Prince Alfred Hospital of Sydney, supported by physicist Edgar H. Booth of the University of Sydney, devised a portable apparatus which "plugged into a lighting point" and in which "One pole was applied to a skin pad soaked in strong salt solution" while the other pole "consisted of a needle insulated except at its point, and was plunged into the appropriate cardiac chamber". "The pacemaker rate was variable from about 80 to 120 pulses per minute, and likewise the voltage variable from 1.5 to 120 volts". In 1928, the apparatus was used to revive a stillborn infant at Crown Street Women's Hospital, Sydney whose heart continued "to beat on its own accord", "at the end of 10 minutes" of stimulation.[35][36]
In 1932, American physiologist Albert Hyman, with the help of his brother, described an electro-mechanical instrument of his own, powered by a spring-wound hand-cranked motor. Hyman himself referred to his invention as an "artificial pacemaker", the term continuing in use to this day.[37][38]
An apparent hiatus in publication of research conducted between the early 1930s and World War II may be attributed to the public perception of interfering with nature by "reviving the dead". For example, "Hyman did not publish data on the use of his pacemaker in humans because of adverse publicity, both among his fellow physicians, and due to newspaper reporting at the time. Lidwell may have been aware of this and did not proceed with his experiments in humans".[36]
Transcutaneous
In 1950, Canadian electrical engineer John Hopps designed and built the first external pacemaker based upon observations by cardio-thoracic surgeons Wilfred Gordon Bigelow and John Callaghan at Toronto General Hospital,[39] although the device was first tested on a dog at the University of Toronto's Banting Institute.[40] A substantial external device using vacuum tube technology to provide transcutaneous pacing, it was somewhat crude and painful to the patient in use and, being powered from an AC wall socket, carried a potential hazard of electrocution of the patient and inducing ventricular fibrillation.
A number of innovators, including Paul Zoll, made smaller but still bulky transcutaneous pacing devices from 1952 using a large rechargeable battery as the power supply.[41]
In 1957, William L. Weirich published the results of research performed at the University of Minnesota. These studies demonstrated the restoration of heart rate, cardiac output and mean aortic pressures in animal subjects with complete heart block through the use of a myocardial electrode.[42]
In 1958 Colombian doctor Alberto Vejarano Laverde and Colombian electrical engineer Jorge Reynolds Pombo constructed an external pacemaker, similar to those of Hopps and Zoll, weighing 45 kg and powered by a 12 volt car lead–acid battery, but connected to electrodes attached to the heart. This apparatus was successfully used to sustain a 70-year-old priest, Gerardo Florez.[43]
The development of the silicon transistor and its first commercial availability in 1956 was the pivotal event that led to rapid development of practical cardiac pacemaking.
Wearable
In 1958, engineer Earl Bakken of Minneapolis, Minnesota, produced the first wearable external pacemaker for a patient of C. Walton Lillehei. This transistorized pacemaker, housed in a small plastic box, had controls to permit adjustment of pacing heart rate and output voltage and was connected to electrode leads which passed through the skin of the patient to terminate in electrodes attached to the surface of the myocardium of the heart.
One of the earliest patients to receive this Lucas pacemaker device was a woman in her early 30s in an operation carried out in 1964 at the Radcliffe Infirmary in Oxford by cardiac surgeon Alf Gunning from South Africa and later Professor Gunning[44][45] who was a student of Christiaan Barnard. This pioneering operation was carried out under the guidance of cardiac consultant Peter Sleight at the Radcliffe Infirmary in Oxford and his cardiac research team at St George's Hospital in London. Sleight later became Professor of Cardiovascular Medicine at Oxford University.[46][47]
Implantable
The first clinical implantation into a human of a fully implantable pacemaker was in 1958 at the Karolinska Institute in Solna, Sweden, using a pacemaker designed by inventor Rune Elmqvist and surgeon Åke Senning (in collaboration with Elema-Schönander AB, later Siemens-Elema AB), connected to electrodes attached to the myocardium of the heart by thoracotomy. The device failed after three hours. A second device was then implanted which lasted for two days. The world's first implantable pacemaker patient, Arne Larsson, went on to receive 26 different pacemakers during his lifetime. He died in 2001, at the age of 86, outliving the inventor as well as the surgeon.[48]
In 1959, temporary transvenous pacing was first demonstrated by Seymour Furman and John Schwedel, whereby the catheter electrode was inserted via the patient's basilic vein.[49]
In February 1960, an improved version of the Swedish Elmqvist design was implanted in Montevideo, Uruguay in the Casmu 1 Hospital by Doctors Orestes Fiandra and Roberto Rubio. That device lasted until the patient died of other ailments, nine months later. The early Swedish-designed devices used rechargeable batteries, which were charged by an induction coil from the outside. It was the first pacemaker implanted in America.
Implantable pacemakers constructed by engineer Wilson Greatbatch entered use in humans from April 1960 following extensive animal testing. The Greatbatch innovation varied from the earlier Swedish devices in using primary cells (mercury battery) as the energy source. The first patient lived for a further 18 months.
The first use of transvenous pacing in conjunction with an implanted pacemaker was by Parsonnet in the United States,[50][51][52] Lagergren in Sweden[53][54] and Jean-Jacques Welti in France[55] in 1962–63. The transvenous, or pervenous, procedure involved incision of a vein into which was inserted the catheter electrode lead under fluoroscopic guidance, until it was lodged within the trabeculae of the right ventricle. This method was to become the method of choice by the mid-1960s.
Cardiothoracic surgeon Leon Abrams and medical engineer Ray Lightwood developed and implanted the first patient-controlled variable-rate heart pacemaker in 1960 at Birmingham University. The first implant took place in March 1960, with two further implants the following month. These three patients made good recoveries and returned to a high quality of life. By 1966, 56 patients had undergone implantation with one surviving for over 5 1⁄2 years.[56][57]
Lithium battery
The preceding implantable devices all suffered from the unreliability and short lifetime of the available primary cell technology which was mainly that of the mercury battery. In the late 1960s, several companies, including ARCO in the USA, developed isotope-powered pacemakers, but this development was overtaken by the development in 1971 of the lithium iodide cell battery by Wilson Greatbatch. Lithium-iodide or lithium anode cells became the standard for future pacemaker designs.
A further impediment to reliability of the early devices was the diffusion of water vapour from the body fluids through the epoxy resin encapsulation affecting the electronic circuitry. This phenomenon was overcome by encasing the pacemaker generator in a hermetically sealed metal case, initially by Telectronics of Australia in 1969 followed by Cardiac Pacemakers Inc of Minneapolis in 1972. This technology, using titanium as the encasing metal, became the standard by the mid-1970s.
On July 9, 1974, Manuel A. Villafaña and Anthony Adducci founders of Cardiac Pacemakers, Inc. (Guidant) in St. Paul, Minnesota, manufactured the world's first pacemaker with a lithium anode and a lithium-iodide electrolyte solid-state battery.[59][60]
Intra-cardial
In 2013, multiple firms announced devices that could be inserted via a leg catheter rather than invasive surgery. The devices are roughly the size and shape of a pill, much smaller than the size of a traditional pacemaker. Once implanted, the device's prongs contact the muscle and stabilize heartbeats. Engineers and scientists are currently working on this type of device.[61] In November 2014 a patient, Bill Pike of Fairbanks, Alaska, received a Medtronic Micra pacemaker in Providence St Vincent Hospital in Portland Oregon. D. Randolph Jones was the EP doctor. In 2014 also St. Jude Medical Inc. announced the first enrollments in the company’s leadless Pacemaker Observational Study evaluating the Nanostim leadless pacing technology. The Nanostim pacemaker received CE marking in 2013. The post-approval implants have occurred in Europe.[62] The European study was recently stopped, after there were reports of six perforations that led to two patient deaths. After investigations St Jude Medical restarted the study.[63] But in the United States this therapy is still not approved by the FDA.[64] While the St Jude Nanostim and the Medtronic Micra are just single-chamber pacemakers it is anticipated that leadless dual-chamber pacing for patients with atrioventricular block will become possible with further development.[65]
Reusable pacemakers
Thousands of pacemakers are removed by funeral home personnel each year all over the world. They have to be removed postmortem from bodies that are going to be cremated to avoid explosions. It is a fairly simple procedure that can be carried out by a mortician. Pacemakers with significant battery life are potentially life-saving devices for people in low and middle income countries (LMICs).[66] The Institute of Medicine, a United States non-governmental organization, has reported that inadequate access to advanced cardiovascular technologies is one of the major contributors to cardiovascular disease morbidity and mortality in LMICs. Ever since the 1970s, multiple studies all over the world have reported on the safety and efficacy of pacemaker reuse. As of 2016, widely acceptable standards for safe pacemaker and ICD reuse have not been developed, and there continue to be legal and regulatory barriers to widespread adoption of medical device reuse.[67]
Manufacturers
Current and prior manufacturers of implantable pacemakers
- Biotronik (Germany)
- Boston Scientific (USA)
- Guidant (USA) (now owned by Boston Scientific)
- Intermedics (USA)
- Lepu Medical (China)
- Medico (Italy)
- Medtronic (USA)
- Sorin Group (Italy) (merged with Cyberonics to form LivaNova)
- St. Jude Medical (USA) (now owned by Abbott Laboratories)
See also
References
- Eich C, Bleckmann A, Paul T (October 2005). "Percussion pacing in a three-year-old girl with complete heart block during cardiac catheterization". Br J Anaesth. 95 (4): 465–7. doi:10.1093/bja/aei209. PMID 16051649.
- "Pacemakers, Patient and Public Information Center : Heart Rhythm Society". Archived from the original on 2010-06-19.
- "The leadless pacemaker: A new era in cardiac pacing". Hospital Healthcare Europe. Archived from the original on 2019-02-02. Retrieved 2019-02-01.
- Bernstein AD, Daubert JC, Fletcher RD, Hayes DL, Lüderitz B, Reynolds DW, Schoenfeld MH, Sutton R (2002). "The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group". Pacing Clin Electrophysiol. 25 (2): 260–4. doi:10.1046/j.1460-9592.2002.00260.x. PMID 11916002. S2CID 12887364.
- Böhm A, Pintér A, Székely A, Préda I (1998). "Clinical Observations with Long-term Atrial Pacing". Pacing Clin Electrophysiol. 21 (1): 246–9. doi:10.1111/j.1540-8159.1998.tb01097.x. PMID 9474681. S2CID 23277568.
- Crick JC (1991). "European Multicenter Prospective Follow-Up Study of 1,002 Implants of a Single Lead VDD Pacing System". Pacing Clin Electrophysiol. 14 (11): 1742–4. doi:10.1111/j.1540-8159.1991.tb02757.x. PMID 1749727. S2CID 698053.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L (2005). "The effect of cardiac resynchronization on morbidity and mortality in heart failure" (PDF). N. Engl. J. Med. 352 (15): 1539–49. doi:10.1056/NEJMoa050496. PMID 15753115.
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH (2005). "Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure". N. Engl. J. Med. 352 (3): 225–37. doi:10.1056/NEJMoa043399. PMID 15659722. S2CID 19118406.
- Ganjehei L, Razavi M, Massumi A (2011). "Cardiac resynchronization therapy: a decade of experience and the dilemma of nonresponders". Texas Heart Institute Journal. 38 (4): 358–60. PMC 3147217. PMID 21841860.
- Sharma, Parikshit S.; Vijayaraman, Pugazhendhi; Ellenbogen, Kenneth A. (2020). "Permanent His bundle pacing: shaping the future of physiological ventricular pacing". Nature Reviews Cardiology. 17 (1): 22–36. doi:10.1038/s41569-019-0224-z. PMID 31249403. S2CID 195698761.
- "Focus on Electrophysiology: His Bundle Pacing: A More Physiologic Alternative For Pacing". American College of Cardiology. April 26, 2019.
- "Anthony Francis Rickards". Heart. 90 (9): 981–2. 2004. doi:10.1136/hrt.2004.045674. PMC 1768450.
- Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A (December 2002). "Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial". JAMA. 288 (24): 3115–23. doi:10.1001/jama.288.24.3115. PMID 12495391.
- Ramsdale, David R. (2012). Cardiac pacing and device therapy. Rao, Archana. London: Springer. ISBN 978-1-4471-2939-4. OCLC 822576869.
- Pena, Rafael E.; Shepard, Richard K.; Ellenbogen, Kenneth A. (December 2006). "How to make a submuscular pocket". Journal of Cardiovascular Electrophysiology. 17 (12): 1381–1383. doi:10.1111/j.1540-8167.2006.00665.x. ISSN 1540-8167. PMID 17081202. S2CID 38032736.
- Marco D, Eisinger G, Hayes DL (November 1992). "Testing of work environments for electromagnetic interference". Pacing Clin Electrophysiol. 15 (11 Pt 2): 2016–22. doi:10.1111/j.1540-8159.1992.tb03013.x. PMID 1279591. S2CID 24234010.
- FDA, "Recently-Approved Devices: Revo MRI SureScan Pacing System". 2013.
- magneticresonancesafetytesting.com
- Larry Husten. "FDA Approves Second Generation MRI-Friendly Pacemaker System From Medtronic". Forbes, 2013-02-13.
- Ferreira, António M; Costa, Francisco; Tralhão, António; Marques, Hugo; Cardim, Nuno; Adragão, Pedro (7 May 2014). "MRI-conditional pacemakers: current perspectives". Medical Devices. 7: 115–124. doi:10.2147/MDER.S44063. PMC 4019608. PMID 24851058.
- "MP3 Headphones Interfere With Implantable Defibrillators, Pacemakers – Beth Israel Deaconess Medical Center". www.bidmc.org. Retrieved 2008-11-10.
- "What is a pacemaker?". HRMReview. Archived from the original on 22 May 2014. Retrieved 22 May 2014.
- Baddour, Larry M.; Epstein, Andrew E.; Erickson, Christopher C.; Knight, Bradley P.; Levison, Matthew E.; Lockhart, Peter B.; Masoudi, Frederick A.; Okum, Eric J.; Wilson, Walter R.; Beerman, Lee B.; Bolger, Ann F.; Estes, N.A. Mark; Gewitz, Michael; Newburger, Jane W.; Schron, Eleanor B.; Taubert, Kathryn A. (26 January 2010). "Update on Cardiovascular Implantable Electronic Device Infections and Their Management". Circulation. 121 (3): 458–477. doi:10.1161/circulationaha.109.192665. PMID 20048212.
- "Heart devices can be turned off near end of life". amednews.com. May 31, 2010.
- Butler, Katy (18 June 2010). "What Broke My Father's Heart". The New York Times.
- Halperin, Daniel; Thomas S. Heydt-Benjamin; Benjamin Ransford; Shane S. Clark; Benessa Defend; Will Morgan; Kevin Fu; Tadayoshi Kohno; William H. Maisel (May 2008). Pacemakers and Implantable Cardiac Defibrillators: Software Radio Attacks and Zero-Power Defenses (PDF). IEEE Symposium on Security and Privacy. Retrieved 2008-08-10.
- "Researchers Develop Personal Firewall Solution for Pacemakers, Insulin Pumps". eSecurityPlanet.com. 2012-04-20. Retrieved 2012-04-20.
- "UOTW #15 - Ultrasound of the Week". Ultrasound of the Week. 26 August 2014. Retrieved 27 May 2017.
- "Risks - Pacemaker - Mayo Clinic". www.mayoclinic.org. Retrieved 2016-12-01.
- Pacemaker-Mediated Tachycardia at eMedicine
- Transvenous Lead Extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management Archived 2014-12-12 at the Wayback Machine Author: Bruce L. Wilkoff, MD. Coauthor(s): Charles J. Love, MD, FHRS, Charles L. Byrd, MD, Maria Grazia Bongiorni, MD, Roger G. Carrillo, MD, FHRS, George H. Crossley, III, MD, FHRS, Laurence M. Epstein, MD, Richard A. Friedman, MD, MBA, FHRS, Charles E. H. Kennergren, MD, PhD, FHRS, Przemyslaw Mitkowski, MD, Raymond H. M. Schaerf, MD, FHRS, Oussama M. Wazni, MD
- Kalavakunta, Jagadeesh Kumar; Gupta, Vishal; Paulus, Basil; Lapenna, William (2014). "An Unusual Cause of Transient Ischemic Attack in a Patient with Pacemaker". Case Reports in Cardiology. 2014: 265759. doi:10.1155/2014/265759. PMC 4008350. PMID 24826308.
- Bernstein AD, Camm AJ, Fisher JD, Fletcher RD, Mead RH, Nathan AW, Parsonnet V, Rickards AF, Smyth NP, Sutton R (1993). "North American Society of Pacing and Electrophysiology policy statement. NASPE/BPEG defibrillator code". Pacing Clin Electrophysiol. 16 (9): 1776–80. doi:10.1111/j.1540-8159.1993.tb01809.x. PMID 7692407. S2CID 72106633.
- McWilliam JA (1889). "Electrical stimulation of the heart in man". Br Med J. 1 (1468): 348–50. doi:10.1136/bmj.1.1468.348. PMC 2154721. PMID 20752595.
- Lidwell M C, "Cardiac Disease in Relation to Anaesthesia" in Transactions of the Third Session, Australasian Medical Congress, Sydney, Australia, Sept. 2–7, 1929, p. 160.
- Mond HG, Sloman JG, Edwards RH (1982). "The first pacemaker". Pacing and Clinical Electrophysiology. 5 (2): 278–82. doi:10.1111/j.1540-8159.1982.tb02226.x. PMID 6176970. S2CID 22049678.
- Aquilina, O (2006). "A brief history of cardiac pacing". Images in Paediatric Cardiology. 8 (2): 17–81. PMC 3232561. PMID 22368662.
- Furman S, Szarka G, Layvand D (2005). "Reconstruction of Hyman's second pacemaker". Pacing Clin Electrophysiol. 28 (5): 446–53. doi:10.1111/j.1540-8159.2005.09542.x. PMID 15869680. S2CID 29138993.
- "John Alexander Hopps fonds". Archival description. Library and Archives Canada. 2008-03-19. Archived from the original on 2020-07-28. Retrieved 16 Sep 2016.
- "IEEE Milestone in Electrical Engineering and Computing". Retrieved September 5, 2009.
- "Paul Maurice Zoll". Harvard Gazette. 19 April 2001.
- Weirich WL, Gott VL, Lillehei CW (1957). "The treatment of complete heart block by the combined use of a myocardial electrode and an artificial pacemaker". Surg Forum. 8: 360–3. PMID 13529629.
- Reynolds, Jorge (March 1988). "The Early History of Cardiac Pacing in Colombia". Pacing and Clinical Electrophysiology. 11 (3): 355–361. doi:10.1111/j.1540-8159.1988.tb05018.x. PMID 2452427. S2CID 20374411.
- "Gunning, Alfred James – Biographical entry – Plarr's Lives of the Fellows Online". Livesonline.rcseng.ac.uk. Retrieved 2013-12-29.
- "Our history". Nuffield Department of Surgical Sciences. University of Oxford. Retrieved 26 October 2020.
- "British Cardiovascular Society". Bcs.com. Archived from the original on 2013-12-12. Retrieved 2013-12-29.
- Record, C O; Sleight, P; Gunning, A J; Kenworthy-Browne, J M; Richings, M (1 November 1971). "Treatment of chronic heart block with the Lucas induction coil pacemaker". Heart. 33 (6): 938–942. doi:10.1136/hrt.33.6.938. PMC 458452. PMID 5120241.
- Altman, Lawrence (18 Jan 2002). "Arne H. W. Larsson, 86; Had First Internal Pacemaker". New York Times. Retrieved 3 March 2014.
- Furman S, Schwedel JB (1959). "An intracardiac pacemaker for Stokes-Adams seizures". N. Engl. J. Med. 261 (5): 943–8. doi:10.1056/NEJM195911052611904. PMID 13825713.
- Parsonnet V (1978). "Permanent transvenous pacing in 1962". Pacing Clin Electrophysiol. 1 (2): 265–8. doi:10.1111/j.1540-8159.1978.tb03472.x. PMID 83641. S2CID 12263609.
- Parsonnet V, Zucker IR, Asa MM (1962). "Preliminary Investigation of the Development of a Permanent Implantable Pacemaker Using an Intracardiac Dipolar Electrode". Clin. Res. 10: 391.
- Parsonnet V, Zucker IR, Gilbert L, Asa M (1962). "An intracardiac bipolar electrode for interim treatment of complete heart block". Am. J. Cardiol. 10 (2): 261–5. doi:10.1016/0002-9149(62)90305-3. PMID 14484083.
- Lagergren H (1978). "How it happened: my recollection of early pacing". Pacing Clin Electrophysiol. 1 (1): 140–3. doi:10.1111/j.1540-8159.1978.tb03451.x. PMID 83610. S2CID 9118036.
- Lagergren H, Johansson L (1963). "Intracardiac stimulation for complete heart block". Acta Chirurgica Scandinavica. 125: 562–566. PMID 13928055.
- Jean Jacques Welti:Biography, Heart Rhythm Foundation
- Blue Plaque Guide
- "University of Birmingham". bhamalumni.org. Archived from the original on 2014-10-06.
- US 3822707
- "Pioneers of the Medical Device Industry". Minnesota Historical Society.
- US US3822707
- "Medtronic's Minimally Invasive Pacemaker the Size of a Multivitamin". Singularity Hub. 2013-12-27. Retrieved 2013-12-29.
- "European Post-Approval Trial for Nanostim". DAIC. 2014-03-18.
- "First-in-Human Data". Medscape. Retrieved 2014-06-19.
- "Leadless Pacing from St. Jude Medical". Archived from the original on 2014-10-29.
- "First Published Data on Leadless Pacemaker Supports Efficacy". Medscape.
- Mazumdar, Tulip (2013-11-19). "British charity calls for re-use of pacemakers abroad". BBC News. Retrieved 2018-07-31.
- Crawford, TC; Eagle, KA (2017). "Reuse of cardiac implantable electronic devices to improve and extend lives: a call to action". Heart Asia. 9 (1): 34–35. doi:10.1136/heartasia-2016-010835. PMC 5278341. PMID 28191825.