Thrombus
A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to prevent bleeding, but can be harmful in thrombosis, when clots obstruct blood flow through healthy blood vessels.
| Thrombus | |
|---|---|
| Other names | Blood clot |
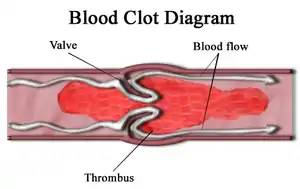 | |
| Diagram of a thrombus (blood clot) that has blocked a blood vessel valve | |
| Specialty | Vascular surgery |
Mural thrombi are thrombi that adhere to the wall of a blood vessel. They occur in large vessels such as the heart and aorta, and can restrict blood flow but usually do not block it entirely. They appear grey-red with alternating light and dark lines (known as lines of Zahn) which represent bands of entrapped white blood cells and red blood cells (darker).
Cause
Virchow's triad describes the pathogenesis of thrombus formation:[1][2]
- Endothelial injury: Injury to the endothelium (interior surface of blood vessel), causing platelet activation and aggregation;
- Common causes include: trauma, smoking, hypertension, atheroma.
- Hemodynamic changes (stasis, turbulence): Blood stasis promotes greater contact between platelets/coagulative factors with vascular endothelium. If rapid blood circulation (e.g., because of tachycardia) occurs within vessels that have endothelial injuries, that creates disordered flow (turbulence) that can lead to the formation of thrombosis;[3]
- Common causes of stasis include anything that leads to prolonged immobility and reduced blood flow such as: trauma/broken bones and extended air travel.
- Hypercoagulability[4] (also called thrombophilia; any disorder of the blood that predisposes to thrombosis);
Disseminated intravascular coagulation (DIC) involves widespread microthrombi formation throughout the majority of the blood vessels. This is due to excessive consumption of coagulation factors and subsequent activation of fibrinolysis using all of the body's available platelets and clotting factors. The end result is hemorrhaging and ischemic necrosis of tissue/organs. Causes are septicaemia, acute leukaemia, shock, snake bites, fat emboli from broken bones, or other severe traumas. DIC may also be seen in pregnant females. Treatment involves the use of fresh frozen plasma to restore the level of clotting factors in the blood, as well as platelets and heparin to prevent further thrombi formation.
Classification
Thrombi are classified into two major groups depending on their location and the relative amount of platelets and red blood cells (RBCs).[5] The two major groups are:
- Arterial or white thrombi (characterized by predominance of platelets)
- Venous or red thrombi (characterized by predominance of red blood cells).
Pathophysiology

A thrombus occurs when the hemostatic process, which normally occurs in response to injury, becomes activated in an uninjured or slightly injured vessel. A thrombus in a large blood vessel will decrease blood flow through that vessel (termed a mural thrombus). In a small blood vessel, blood flow may be completely cut off (termed an occlusive thrombus), resulting in death of tissue supplied by that vessel. If a thrombus dislodges and becomes free-floating, it is considered an embolus.
Some of the conditions which increase the risk of blood clots developing include atrial fibrillation (a form of cardiac arrhythmia), heart valve replacement, a recent heart attack (also known as a myocardial infarction), extended periods of inactivity (see deep venous thrombosis), and genetic or disease-related deficiencies in the blood's clotting abilities.
Formation
Platelet activation occurs through injuries that damage the endothelium of the blood vessels, exposing the enzyme called factor VII, a protein normally circulating within the vessels, to the tissue factor, which is a protein encoded by the F3 gene. The platelet activation can potentially cause a cascade, eventually leading to the formation of the thrombus.[6] This process is regulated through thromboregulation.
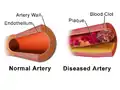 Illustration Comparing Normal Artery vs Diseased Artery with a Blood Clot
Illustration Comparing Normal Artery vs Diseased Artery with a Blood Clot
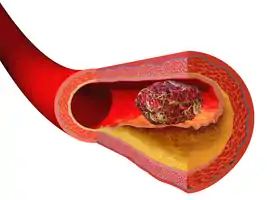 Illustration depicting thrombus formation over arterial plaque.
Illustration depicting thrombus formation over arterial plaque.
Prevention and treatment
Anticoagulants are drugs used to prevent the formation of blood clots, reducing the risk of stroke, heart attack and pulmonary embolism. Heparin and warfarin are used to inhibit the formation and growth of existing thrombi, with the former used for acute anticoagulation while the latter is used for long-term anticoagulation.[2] The mechanism of action of heparin and warfarin are different as they work on different pathways of the coagulation cascade.[7] Heparin works by binding to and activating the enzyme inhibitor antithrombin III, an enzyme that acts by inactivating thrombin and factor Xa.[7] In contrast, warfarin works by inhibiting vitamin K epoxide reductase, an enzyme needed to synthesize vitamin K dependent clotting factors II, VII, IX, and X.[7][8] Bleeding time with heparin and warfarin therapy can be measured with the partial thromboplastin time (PTT) and prothrombin time (PT), respectively.[8]
Once clots have formed, other drugs can be used to promote thrombolysis or clot breakdown. Streptokinase, an enzyme produced by streptococcal bacteria, is one of the oldest thrombolytic drugs.[8] This drug can be administered intravenously to dissolve blood clots in coronary vessels. However, streptokinase causes systemic fibrinolytic state and can lead to bleeding problems. Tissue plasminogen activator (tPA) is a different enzyme that promotes the degradation of fibrin in clots but not free fibrinogen.[8] This drug is made by transgenic bacteria and converts plasminogen into the clot-dissolving enzyme, plasmin.[9] Recent research indicates that tPA could have toxic effects in the central nervous system. In cases of severe stroke, tPA can cross the blood-brain barrier and enter interstitial fluid, where it then increases excitotoxicity, potentially affecting permeability of the blood-brain barrier,[10] and causing cerebral hemorrhage.[11]
There are also some anticoagulants that come from animals that work by dissolving fibrin. For example, Haementeria ghilianii, an Amazon leech, produces an enzyme called hementin from its salivary glands.[12]
Prognosis
Thrombus formation can have one of four outcomes: propagation, embolization, dissolution, and organization and recanalization.[13]
- Propagation of a thrombus occurs towards the direction of the heart and involves the accumulation of additional platelets and fibrin. This means that it is anterograde in veins or retrograde in arteries.
- Embolization occurs when the thrombus breaks free from the vascular wall and becomes mobile, thereby traveling to other sites in the vasculature. A venous embolus (mostly from deep vein thrombosis in the lower limbs) will travel through the systemic circulation, reach the right side of the heart, and travel through the pulmonary artery, resulting in a pulmonary embolism. Arterial thrombosis resulting from hypertension or atherosclerosis can become mobile and the resulting emboli can occlude any artery or arteriole downstream of the thrombus formation. This means that cerebral stroke, myocardial infarction, or any other organ can be affected.
- Dissolution occurs when the fibrinolytic mechanisms break up the thrombus and blood flow is restored to the vessel. This may be aided by fibrinolytic drugs such as Tissue Plasminogen Activator (tPA) in instances of coronary artery occlusion. The best response to fibrinolytic drugs is within a couple of hours, before the fibrin meshwork of the thrombus has been fully developed.
- Organization and recanalization involves the ingrowth of smooth muscle cells, fibroblasts and endothelium into the fibrin-rich thrombus. If recanalization proceeds it provides capillary-sized channels through the thrombus for continuity of blood flow through the entire thrombus but may not restore sufficient blood flow for the metabolic needs of the downstream tissue.[1]
See also
- Thrombosis
- Embolism
- Thrombolysis ("destruction of clot")
- Thrombogenicity (the tendency to clot)
- National Blood Clot Alliance
- Prothrombin time
- Hemorrhoid
References
- Kumar, Vinay; Abbas, Abul; Aster, Jon (2014). Robbins & Cotran Pathologic Basis of Disease (9th ed.). Philadelphia, PA: Elsevier. ISBN 9781455726134. OCLC 879416939.
- "Venous thromboembolism (VTE) | McMaster Pathophysiology Review". www.pathophys.org. Retrieved 2018-11-03.
- Kushner, Abigail; West, William P.; Pillarisetty, Leela Sharath (2020), "Virchow Triad", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30969519, retrieved 2020-06-18
- Ataga KI (10 May 2020). "Hypercoagulability and thrombotic complications in hemolytic anemias". Haematologica. 94 (11): 1481–1484. doi:10.3324/haematol.2009.013672. PMC 2770956. PMID 19880774.
- "Thrombus Formation - Virchow's triad & Types of Thrombi". www.thrombosisadviser.com. Bayer AG. Retrieved 20 March 2020.
- Furie, Bruce; Furie, Barbara (2008). "Mechanisms of Thrombus Formation". The New England Journal of Medicine. 359 (9): 938–49. doi:10.1056/NEJMra0801082. PMID 18753650.
- Harter, K.; Levine, M.; Henderson, S. O. (2015). "Anticoagulation Drug Therapy: A Review". The Western Journal of Emergency Medicine. 16 (1): 11–17. doi:10.5811/westjem.2014.12.22933. PMC 4307693. PMID 25671002.
- Whalen, Karen; Finkel, Richard S.; Panavelil, Thomas A. (2015). Lippincott Illustrated Reviews: Pharmacology (6th ed.). Philadelphia: Wolters Kluwer. ISBN 9781451191776. OCLC 881019575.
- Saladin, Kenneth S. (2012). Anatomy & Physiology: The Unity of Form and Function (6th ed.). New York, NY: McGraw-Hill. p. 710. ISBN 978-0-07-337825-1.
- Fredriksson, L.; Lawrence, D. A.; Medcalf, R. L. (2016). "TPA modulation of the blood-brain barrier: A unifying explanation for the pleiotropic effects of tPA in the CNS?". Seminars in Thrombosis and Hemostasis. 43 (2): 154–168. doi:10.1055/s-0036-1586229. PMC 5848490. PMID 27677179.
- Medcalf, R. (2011). "Plasminogen activation-based thrombolysis for ischaemic stroke: the diversity of targets may demand new approaches". Current Drug Targets. 12 (12): 1772–1781. doi:10.2174/138945011797635885. PMID 21707475.
- Budzynski, A. Z. (1991). "Interaction of hementin with fibrinogen and fibrin". Blood Coagulation & Fibrinolysis. 2 (1): 149–52. doi:10.1097/00001721-199102000-00022. PMID 1772982.
- Kumar, Vinay; et al. (2007). Robbins Basic Pathology (8th ed.). Philadelphia: Saunders/Elsevier. ISBN 978-1-4160-2973-1.
External links
| Look up thrombus or clot in Wiktionary, the free dictionary. |
- Treatment and Symptoms of Blood Clots -- MDhealthnetwork.org, Medical Information Resource, 1999
- North American Thrombosis Forum - NATF is a nonprofit organization dedicated to promoting thrombosis research, prevention and education, public policy, and advocacy outreach.
- Muscle Relaxing Drugs Can Reduce Lethal Blood Clots
- Air Pollution Triggers Blood Clots - US Study.
- The National Alliance for Thrombosis and Thrombophilia - has extensive stories from patients and family members on living with Thrombosis and Thrombophilia, assembled frequently asked questions and created publications addressing specific blood clot issues.
| Classification |
|---|