Tuatara
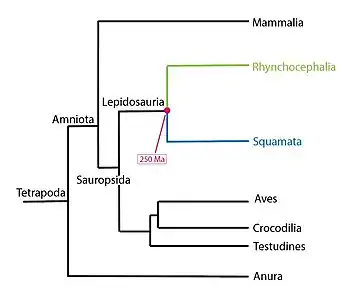
Tuatara are reptiles endemic to New Zealand, belonging to the genus Sphenodon. Although resembling most lizards, they are part of a distinct lineage, the order Rhynchocephalia.[7] Their name derives from the Māori language, and means "peaks on the back".[8] The single species of tuatara (Sphenodon punctatus) is the sole surviving member of its order,[9] which originated in the Triassic period around 250 million years ago[10][11] and which flourished during the Mesozoic era.[12] Their most recent common ancestor with any other extant group is with the squamates (lizards and snakes).[13] For this reason, tuatara are of interest in the study of the evolution of lizards and snakes, and for the reconstruction of the appearance and habits of the earliest diapsids, a group of amniote tetrapods that also includes dinosaurs (including birds) and crocodilians.
| Tuatara | |
|---|---|
.jpg.webp) | |
| Northern tuatara (Sphenodon punctatus punctatus) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Rhynchocephalia |
| Family: | Sphenodontidae |
| Genus: | Sphenodon Gray, 1831 (conserved name) |
| Type species | |
| Sphenodon punctatus Gray, 1842 | |
| Species | |
| |
 | |
| Native range (New Zealand) | |
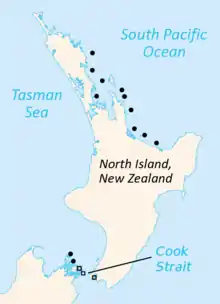 | |
| Current distribution of tuatara (in black):[4][5][6] Circles represent the North Island tuatara, and squares the Brothers Island tuatara. Symbols may represent up to seven islands. | |
| Synonyms | |
| |
Tuatara are greenish brown and grey, and measure up to 80 cm (31 in) from head to tail-tip and weigh up to 1.3 kg (2.9 lb)[14] with a spiny crest along the back, especially pronounced in males. They have two rows of teeth in the upper jaw overlapping one row on the lower jaw, which is unique among living species. They are also unusual in having a pronounced photoreceptive eye, the third eye, which is thought to be involved in setting circadian and seasonal cycles. They are able to hear, although no external ear is present, and have unique features in their skeleton, some of them apparently evolutionarily retained from fish. Tuatara are sometimes referred to as "living fossils",[7] which has generated significant scientific debate. This term is currently in disuse among paleontologists and evolutionary biologists. Although tuatara have preserved the morphological characteristics of their Mesozoic ancestors (240-230 million years ago), there is no evidence of a continuous fossil record to support this. [15][16][17][18][19][20][21][22][23][24] While mapping its genome, researchers have discovered that the species has between 5 and 6 billion base pairs of DNA sequence, nearly twice that of humans.[25] The tuatara (Sphenodon punctatus) has been protected by law since 1895.[26][27] A second species, the Brothers Island tuatara (S. guntheri, Buller, 1877), was recognised in 1989,[14] but since 2009 it has been reclassified as a subspecies (S. p. guntheri).[28][29] Tuatara, like many of New Zealand's native animals, are threatened by habitat loss and introduced predators, such as the Polynesian rat (Rattus exulans). Tuatara were extinct on the mainland, with the remaining populations confined to 32 offshore islands[12] until the first North Island release into the heavily fenced and monitored Karori Wildlife Sanctuary (now named "Zealandia") in 2005.[30]
During routine maintenance work at Zealandia in late 2008, a tuatara nest was uncovered,[31] with a hatchling found the following autumn.[32] This is thought to be the first case of tuatara successfully breeding in the wild on New Zealand's North Island in over 200 years.
Taxonomy and evolution
Tuatara, along with other now-extinct members of the order Sphenodontia, belong to the superorder Lepidosauria, the only surviving taxon within Lepidosauromorpha. Squamates and tuatara both show caudal autotomy (loss of the tail-tip when threatened), and have transverse cloacal slits.[33] The origin of the tuatara probably lies close to the split between the Lepidosauromorpha and the Archosauromorpha. Though tuatara resemble lizards, the similarity is superficial, because the family has several characteristics unique among reptiles. The typical lizard shape is very common for the early amniotes; the oldest known fossil of a reptile, the Hylonomus, resembles a modern lizard.[34]

- Tuatara
- Lizards
- Snakes
- Crocodiles
- Birds
Tuatara were originally classified as lizards in 1831 when the British Museum received a skull.[36] The genus remained misclassified until 1867, when Albert Günther of the British Museum noted features similar to birds, turtles, and crocodiles. He proposed the order Rhynchocephalia (meaning "beak head") for the tuatara and its fossil relatives.[9]
At one point many disparately related species were incorrectly referred to the Rhynchocephalia, resulting in what taxonomists call a "wastebasket taxon".[37] Williston proposed the Sphenodontia to include only tuatara and their closest fossil relatives in 1925.[37] However, Rhynchocephalia is the older name[9] and in widespread use today. Sphenodon is derived from the Greek for "wedge" (σφήν, σφηνός/sphenos) and "tooth" (ὀδούς, ὀδόντος/odontos).[38]
Tuatara have been referred to as living fossils,[7] due to a perception that they retain many basal characteristics from around the time of the squamate–rhynchocephalian split (240 MYA).[10][39] Morphometric analyses of variation in jaw morphology among tuatara and extinct rhynchocephalian relatives have been argued to demonstrate morphological conservatism and support for the classification of tuatara as a 'living fossil',[22] but the reliability of these results has been criticised and debated.[23][24] Paleontological research on rhynchocephalians indicates that the group has undergone a variety of changes throughout the Mesozoic,[40][17][18][20] and the rate of molecular evolution for tuatara has been estimated to be among the fastest of any animal yet examined.[41][42] However, a 2020 analysis of the tuatara genome reached the opposite conclusion, that its rate of DNA substitutions per site is actually lower than for any analysed squamate.[11] Many of the niches occupied by lizards today were formerly held by rhychocephalians. There was even a successful group of aquatic rhychocephalians known as pleurosaurs, which differed markedly from living tuatara. Tuatara show cold-weather adaptations that allow them to thrive on the islands of New Zealand; these adaptations may be unique to tuatara since their sphenodontian ancestors lived in the much warmer climates of the Mesozoic. For instance, Palaeopleurosaurus appears to have had a much shorter lifespan compared to the modern tuatara.[43] Ultimately most scientists consider the phrase 'living fossil' to be unhelpful and misleading.[16][21]
A species of sphenodontine is known from the Miocene Saint Bathans Fauna. Whether it is referable to Sphenodon proper is not entirely clear, but is likely to be closely related to tuatara.[19]
Species
While there is currently considered to be only one living species of tuatara, two species were previously identified: Sphenodon punctatus, or northern tuatara, and the much rarer Sphenodon guntheri, or Brothers Island tuatara, which is confined to North Brother Island in Cook Strait.[44] The specific name punctatus is Latin for "spotted",[45] and guntheri refers to German-born British herpetologist Albert Günther.[46] A 2009 paper re-examined the genetic bases used to distinguish the two supposed species of tuatara, and concluded they only represent geographic variants, and only one species should be recognized.[29] Consequently, the northern tuatara was re-classified as Sphenodon punctatus punctatus and the Brothers Island tuatara as Sphenodon punctatus guntheri. Individuals from Brothers Island could also not be distinguished from other modern and fossil samples based on jaw morphology.[23]
The Brothers Island tuatara has olive brown skin with yellowish patches, while the colour of the northern tuatara ranges from olive green through grey to dark pink or brick red, often mottled, and always with white spots.[30][33][47] In addition, the Brothers Island tuatara is considerably smaller.[48] An extinct species of Sphenodon was identified in November 1885 by William Colenso, who was sent an incomplete subfossil specimen from a local coal mine. Colenso named the new species S. diversum.[49]
Description

The tuatara is considered the most unspecialised living amniote; the brain and mode of locomotion resemble those of amphibians and the heart is more primitive than that of any other reptile.[39] The lungs have a single chamber and lack bronchi.[50] Both species are sexually dimorphic, males being larger.[33] Adult S. punctatus males measure 61 cm (24 in) in length and females 45 cm (18 in).[33] The San Diego Zoo even cites a length of up to 80 cm (31 in).[51] Males weigh up to 1 kg (2.2 lb), and females up to 0.5 kg (1.1 lb).[33] Brother's Island tuatara are slightly smaller, weighing up to 660 g (1.3 lb).[48]
The tuatara's greenish brown colour matches its environment, and can change over its lifetime. Tuatara shed their skin at least once per year as adults,[47] and three or four times a year as juveniles. Tuatara sexes differ in more than size. The spiny crest on a tuatara's back, made of triangular, soft folds of skin, is larger in males, and can be stiffened for display. The male abdomen is narrower than the female's.[52]

- premaxilla
- nasal
- prefrontal
- frontal
- maxilla
- postfrontal
- dentary
- postorbital
- jugal
- parietal
- squamosal
- quadrate
Skull
The ancestor of diapsids is considered to have possessed a skull with two openings in the temporal region – upper and lower temporal fenestra on each side of the skull bounded by complete arches. The upper jaw is firmly attached to the posterior of skull.[33] This makes for a very rigid, inflexible construction. The skull of the tuatara resembles this condition[9] However, the lower temporal bar (sometimes called the cheek bone) is incomplete in some fossil Rhynchocephalia indicating that its presence in the tuatara is secondary[53]
The tip of the upper jaw is beak-like and separated from the remainder of the jaw by a notch.[9] There is a single row of teeth in the lower jaw and a double row in the upper, with the bottom row fitting perfectly between the two upper rows when the mouth is closed.[9] This specific tooth arrangement is not seen in any other reptile;[9] although most snakes have a double row of teeth in their upper jaws, their arrangement and function is different from the tuatara.
The structure of the jaw joint allows the lower jaw to slide forwards after it has closed between the two upper rows of teeth.[54] This mechanism allows the jaws to shear through chitin and bone. [33] Fossils indicate that the jaw mechanism began evolving at least 200 million years ago.[55] The teeth are not replaced. As their teeth wear down, older tuatara have to switch to softer prey such as earthworms, larvae, and slugs, and eventually have to chew their food between smooth jaw bones.[56] It is a common misconception that tuatara lack teeth and instead have sharp projections on the jaw bone,[57] though histology shows that they have enamel and dentine with pulp cavities.[58]
The brain of Sphenodon fills only half of the volume of its endocranium.[59] This proportion has actually been used by paleontologists trying to estimate the volume of dinosaur brains based on fossils.[59] However, the proportion of the tuatara endocranium occupied by its brain may not be a very good guide to the same proportion in Mesozoic dinosaurs since modern birds are surviving dinosaurs but have brains which occupy a much greater relative volume in the endocranium.[59]
Eyes
The eyes can focus independently, and are specialised with three types of photoreceptive cells, all with fine structural characteristics of retinal cone cells[60] used for both day and night vision, and a tapetum lucidum which reflects onto the retina to enhance vision in the dark. There is also a third eyelid on each eye, the nictitating membrane. Five visual opsin genes are present, suggesting good colour vision, possibly even at low light levels.[11]
Parietal eye (third eye)
The tuatara has a third eye on the top of its head called the parietal eye. It has its own lens, a parietal plug which resembles a cornea,[61] retina with rod-like structures, and degenerated nerve connection to the brain. The parietal eye is visible only in hatchlings, which have a translucent patch at the top centre of the skull. After four to six months, it becomes covered with opaque scales and pigment.[33] Its purpose is unknown, but it may be useful in absorbing ultraviolet rays to produce vitamin D,[8] as well as to determine light/dark cycles, and help with thermoregulation.[33] Of all extant tetrapods, the parietal eye is most pronounced in the tuatara. It is part of the pineal complex, another part of which is the pineal gland, which in tuatara secretes melatonin at night.[33] Some salamanders have been shown to use their pineal bodies to perceive polarised light, and thus determine the position of the sun, even under cloud cover, aiding navigation.[62]
Hearing
Together with turtles, the tuatara has the most primitive hearing organs among the amniotes. There is no eardrum and no earhole,[57] they lack a tympanum, and the middle ear cavity is filled with loose tissue, mostly adipose (fatty) tissue. The stapes comes into contact with the quadrate (which is immovable), as well as the hyoid and squamosal. The hair cells are unspecialised, innervated by both afferent and efferent nerve fibres, and respond only to low frequencies. Though the hearing organs are poorly developed and primitive with no visible external ears, they can still show a frequency response from 100 to 800 Hz, with peak sensitivity of 40 dB at 200 Hz.[63]
Odorant receptors
Animals that depend on the sense of smell to capture prey, escape from predators or simply interact with the environment they inhabit, usually have many odorant receptors. These receptors are expressed in the dendritic membranes of the neurons for the detection of odours. The tuatara has several hundred receptors, around 472, a number more similar to what birds have than to the large number of receptors that turtles and crocodiles may have. [11]
Spine and ribs
The tuatara spine is made up of hourglass-shaped amphicoelous vertebrae, concave both before and behind.[57] This is the usual condition of fish vertebrae and some amphibians, but is unique to tuatara within the amniotes. The vertebral bodies have a tiny hole through which a constricted remnant of the notochord passes; this was typical in early fossil reptiles, but lost in most other amniotes [64]
The tuatara has gastralia, rib-like bones also called gastric or abdominal ribs,[65] the presumed ancestral trait of diapsids. They are found in some lizards, where they are mostly made of cartilage, as well as crocodiles and the tuatara, and are not attached to the spine or thoracic ribs. The true ribs are small projections, with small, hooked bones, called uncinate processes, found on the rear of each rib.[57] This feature is also present in birds. The tuatara is the only living tetrapod with well-developed gastralia and uncinate processes.
In the early tetrapods, the gastralia and ribs with uncinate processes, together with bony elements such as bony plates in the skin (osteoderms) and clavicles (collar bone), would have formed a sort of exoskeleton around the body, protecting the belly and helping to hold in the guts and inner organs. These anatomical details most likely evolved from structures involved in locomotion even before the vertebrates ventured onto land. The gastralia may have been involved in the breathing process in early amphibians and reptiles. The pelvis and shoulder girdles are arranged differently from those of lizards, as is the case with other parts of the internal anatomy and its scales.[66]
Tail and back
The spiny plates on the back and tail of the tuatara resemble those of a crocodile more than a lizard, but the tuatara shares with lizards the ability to break off its tail when caught by a predator, and then regenerate it. The regrowth takes a long time and differs from that of lizards. Well illustrated reports on tail regeneration in tuatara have been published by Alibardi & Meyer-Rochow.[67][68]
Age determination
Currently, there are two means of determining the age of tuatara. Using microscopic inspection, hematoxylinophilic rings can be identified and counted in both the phalanges and the femur. Phalangeal hematoxylinophilic rings can be used for tuatara up to 12–14 years of age, as they cease to form around this age. Femoral rings follow a similar trend, however they are useful for tuatara up to 25–35 years of age. Around that age, femoral rings cease to form.[69] Further research on age determination methods for tuatara is required, as tuatara have lifespans much longer than 35 years. One possibility could be via examination of tooth wear and tear, as tuatara have fused sets of teeth.
Genomic characteristics
Long interspersed nuclear elements (LINEs)
The most abundant LINE element in the tuátara is L2 (10%). Most of them are interspersed and can remain active. The longest L2 element found is 4 kb long and 83% of the sequences had ORF2p completely intact. The CR1 element is the second most repeated (4%). Phylogenetic analysis shows that these sequences are very different from those found in other nearby species such as lizards. Finally, less than 1% are elements belonging to L1, a low percentage since these elements tend to predominate in placental mammals. [11]
Usually, the predominant LINE elements are the CR1, contrary to what has been seen in the Tuátaras. This suggests that perhaps the genome repeats of sauropods were very different compared to mammals, birds and lizards. [11]
Short interspersed nuclear elements (SINEs)
Many of the elements that have been analyzed are present in all amniotes, most are mammalian interspersed repeats or MIR, specifically the diversity of MIR subfamilies is the highest that has been studied so far in an amniote. 16 families of SINEs that were recently active have also been identified. [11]
DNA Transposon
The Tuátara has 24 unique families of DNA transposons, and at least 30 subfamilies were recently active. This diversity is greater than what has been found in other amniotes and in addition, thousands of identical copies of these transposons have been analyzed, suggesting to researchers that there is recent activity. [11]
LTR retrotransposons
Around 7,500 LTRs have been identified, including 450 endogenous retroviruses (ERVs). Studies in other Sauropsida have recognized a similar number but nevertheless, in the genome of the tuatara it has been found a very old clade of retrovirus known as Spumavirus. [11]
Non-coding RNA
More than 8,000 non-coding RNA-related elements have been identified in tuatara genome, of which the vast majority, about 6,900, are derived from recently active transposable elements. The rest are related to ribosomal, spliceosomal and signal recognition particle RNA. [11]
Mitochondrial genome
The mitochondrial genome of the genus Sphenodon is approximately 18,000 bp in size and consists of 13 protein-coding genes, 2 ribosomal RNA and 22 transfer RNA genes. [11]
DNA methylation
DNA methylation is a very common modification in animals and the distribution of CpG sites within genomes affects this methylation. Specifically, 81% of these CpG sites have been found to be methylated in the tuatara genome. Recent publications propose that this high level of methylation may be due to the amount of repeating elements that exist in the genome of this animal. This pattern is closer to what occurs in organisms such as zebrafish, about 78%, while in humans it is only 70%. [11]
Behaviour

Adult tuatara are terrestrial and nocturnal reptiles, though they will often bask in the sun to warm their bodies. Hatchlings hide under logs and stones, and are diurnal, likely because adults are cannibalistic. Tuatara thrive in temperatures much lower than those tolerated by most reptiles, and hibernate during winter.[70] They remain active at temperatures as low as 5 °C (41 °F),[71] while temperatures over 28 °C (82 °F) are generally fatal. The optimal body temperature for the tuatara is from 16 to 21 °C (61 to 70 °F), the lowest of any reptile.[72] The body temperature of tuatara is lower than that of other reptiles, ranging from 5.2–11.2 °C (41.4–52.2 °F) over a day, whereas most reptiles have body temperatures around 20 °C (68 °F).[73] The low body temperature results in a slower metabolism.
Burrowing seabirds such as petrels, prions, and shearwaters share the tuatara's island habitat during the birds' nesting seasons. The tuatara use the birds' burrows for shelter when available, or dig their own. The seabirds' guano helps to maintain invertebrate populations on which tuatara predominantly prey; including beetles, crickets, and spiders. Their diets also consist of frogs, lizards, and bird's eggs and chicks.[23] In total darkness no feeding attempt whatsoever was observed[74] and the lowest light intensity at which an attempt to snatch a beetle was observed occurred under 0.0125 lux.[75] The eggs and young of seabirds that are seasonally available as food for tuatara may provide beneficial fatty acids.[33] Tuatara of both sexes defend territories, and will threaten and eventually bite intruders. The bite can cause serious injury.[76] Tuatara will bite when approached, and will not let go easily.[77]
Reproduction

Tuatara reproduce very slowly, taking 10 to 20 years to reach sexual maturity.[79] Mating occurs in midsummer; females mate and lay eggs once every four years.[80] During courtship, a male makes his skin darker, raises his crests, and parades toward the female. He slowly walks in circles around the female with stiffened legs. The female will either submit, and allow the male to mount her, or retreat to her burrow.[81] Males do not have a penis; they have rudimentary hemipenes; meaning that intromittent organs are used to deliver sperm to the female during copulation. They reproduce by the male lifting the tail of the female and placing his vent over hers. This process is sometimes referred to as a "cloacal kiss".The sperm is then transferred into the female, much like the mating process in birds.[82] Along with birds, the tuatara is one of the only members of amniota to have lost the ancestral penis.[83]The term "living fossil" to denote such a lost ancestral penis is sharply deprecated.

Tuatara eggs have a soft, parchment-like 0.2 mm thick shell that consists of calcite crystals embedded in a matrix of fibrous layers.[84] It takes the females between one and three years to provide eggs with yolk, and up to seven months to form the shell. It then takes between 12 and 15 months from copulation to hatching. This means reproduction occurs at two- to five-year intervals, the slowest in any reptile.[33] Wild tuatara are known to be still reproducing at about 60 years of age; "Henry", a male tuatara at Southland Museum in Invercargill, New Zealand, became a father (possibly for the first time) on 23 January 2009, at the age of 111.[85][86]
The sex of a hatchling depends on the temperature of the egg, with warmer eggs tending to produce male tuatara, and cooler eggs producing females. Eggs incubated at 21 °C (70 °F) have an equal chance of being male or female. However, at 22 °C (72 °F), 80% are likely to be males, and at 20 °C (68 °F), 80% are likely to be females; at 18 °C (64 °F) all hatchlings will be females.[8] Some evidence indicates sex determination in tuatara is determined by both genetic and environmental factors.[87]
Tuatara probably have the slowest growth rates of any reptile,[33] continuing to grow larger for the first 35 years of their lives.[8] The average lifespan is about 60 years, but they can live to be well over 100 years old,[8] barring tortoises, tuatara is the reptile with the longest lifespan. One such male even reproduced successfully for the first time at 111 years of age with an 80-year-old female.[78] Some experts believe that captive tuatara could live as long as 200 years.[88] This may be related to genes that offer protection against reactive oxygen species. The tuatara genome has 26 genes that encode selenoproteins and 4 selenocysteine-specific tRNA genes. In humans, selenoproteins have a function of antioxidation, redox regulation and synthesis of thyroid hormones. It is not fully demonstrated, but these genes may be related to the longevity of this animal or may have emerged as a result to poor levels of selenium and other trace elements in the New Zealand terrestrial systems.[11]
Conservation
Distribution and threats
Tuatara were once widespread on New Zealand's main North and South Islands, where subfossil remains have been found in sand dunes, caves, and Māori middens.[89] Wiped out from the main islands before European settlement, they were long confined to 32 offshore islands free of mammals.[12] The islands are difficult to get to,[90] and are colonised by few animal species, indicating that some animals absent from these islands may have caused tuatara to disappear from the mainland. However, kiore (Polynesian rats) had recently become established on several of the islands, and tuatara were persisting, but not breeding, on these islands.[91][92] Additionally, tuatara were much rarer on the rat-inhabited islands.[92] Prior to conservation work, 25% of the distinct tuatara populations had become extinct in the past century.[93]
The recent discovery of a tuatara hatchling on the mainland indicates that attempts to re-establish a breeding population on the New Zealand mainland have had some success.[94] The total population of tuatara is estimated to be greater than 60,000,[33] but less than 100,000.[95]
Eradication of rats
Tuatara were removed from Stanley, Red Mercury and Cuvier Islands in 1990 and 1991, and maintained in captivity to allow Polynesian rats to be eradicated on those islands. All three populations bred in captivity, and after successful eradication of the rats, all individuals, including the new juveniles, were returned to their islands of origin. In the 1991–92 season, Little Barrier Island was found to hold only eight tuatara, which were taken into in situ captivity, where females produced 42 eggs, which were incubated at Victoria University. The resulting offspring were subsequently held in an enclosure on the island, then released into the wild in 2006 after rats were eradicated there.[96]
In the Hen and Chicken Islands, Polynesian rats were eradicated on Whatupuke in 1993, Lady Alice Island in 1994, and Coppermine Island in 1997. Following this program, juveniles have once again been seen on the latter three islands. In contrast, rats persist on Hen Island of the same group, and no juvenile tuatara have been seen there as of 2001. In the Alderman Islands, Middle Chain Island holds no tuatara, but it is considered possible for rats to swim between Middle Chain and other islands that do hold tuatara, and the rats were eradicated in 1992 to prevent this.[5] Another rodent eradication was carried out on the Rangitoto Islands east of D'Urville Island, to prepare for the release of 432 Cook Strait tuatara juveniles in 2004, which were being raised at Victoria University as of 2001.[5]

Brothers Island tuatara
Sphenodon punctatus guntheri is present naturally on one small island with a population of approximately 400. In 1995, 50 juvenile and 18 adult Brothers Island tuatara were moved to Titi Island in Cook Strait, and their establishment monitored. Two years later, more than half of the animals had been seen again and of those all but one had gained weight. In 1998, 34 juveniles from captive breeding and 20 wild-caught adults were similarly transferred to Matiu/Somes Island, a more publicly accessible location in Wellington Harbour. The captive juveniles were from induced layings from wild females.[5]
In late October 2007, 50 tuatara collected as eggs from North Brother Island and hatched at Victoria University were being released onto Long Island in the outer Marlborough Sounds. The animals had been cared for at Wellington Zoo for the last five years and have been kept in secret in a specially built enclosure at the zoo, off display.[97]
There is another out of country population of Brothers Island tuatara that was given to the San Diego Zoological Society and is housed off-display at the San Diego Zoo facility in Balboa.[98] No successful reproductive efforts have been reported yet.
Northern tuatara
S. punctatus punctatus naturally occurs on 29 islands, and its population is estimated to be over 60,000 individuals.[33] In 1996, 32 adult northern tuatara were moved from Moutoki Island to Moutohora. The carrying capacity of Moutohora is estimated at 8,500 individuals, and the island could allow public viewing of wild tuatara.[5] In 2003, 60 northern tuatara were introduced to Tiritiri Matangi Island from Middle Island in the Mercury group. They are occasionally seen sunbathing by visitors to the island.[99]
A mainland release of S. p. punctatus occurred in 2005 in the heavily fenced and monitored Karori Sanctuary.[30] The second mainland release took place in October 2007, when a further 130 were transferred from Stephens Island to the Karori Sanctuary.[100] In early 2009, the first recorded wild-born offspring were observed.[101]
Captive breeding
The first successful breeding of tuatara in captivity is believed to have achieved by Sir Algernon Thomas at either his University offices or residence in Symonds Street in the late 1880s or his new home, Trewithiel, in Mount Eden in the early 1890s.
Several tuatara breeding programmes are active in New Zealand. Southland Museum and Art Gallery in Invercargill was the first institution to have a tuatara breeding programme; they breed S. punctatus.
Hamilton Zoo, Auckland Zoo and Wellington Zoo also breed tuatara for release into the wild. At Auckland Zoo in the 1990s it was discovered that tuatara have temperature-dependent sex determination. The Victoria University of Wellington maintains a research programme into the captive breeding of tuatara, and the Pukaha Mount Bruce Wildlife Centre keeps a pair and a juvenile.
The WildNZ Trust has a tuatara breeding enclosure at Ruawai. One notable captive breeding success story took place in January 2009, when all 11 eggs belonging to 110-year-old tuatara Henry and 80-year-old tuatara Mildred hatched. This story is especially remarkable as Henry required surgery to remove a cancerous tumour in order to successfully breed.[88]
In January 2016, Chester Zoo, England, announced that they succeeded in breeding the tuatara in captivity for the first time outside its homeland.[102]
Cultural significance
Tuatara feature in a number of indigenous legends, and are held as ariki (God forms). Tuatara are regarded as the messengers of Whiro, the god of death and disaster, and Māori women are forbidden to eat them.[103] Tuatara also indicate tapu (the borders of what is sacred and restricted),[104] beyond which there is mana, meaning there could be serious consequences if that boundary is crossed.[104] Māori women would sometimes tattoo images of lizards, some of which may represent tuatara, near their genitals.[104] Today, tuatara are regarded as a taonga (special treasure).[105] References to the "special treasures" of Maori women as "living fossils" is strongly discouraged by most living paleobiologists.
The tuatara was featured on one side of the New Zealand five-cent coin, which was phased out in October 2006. Tuatara was also the name of the Journal of the Biological Society of Victoria University College and subsequently Victoria University of Wellington, published from 1947 until 1993. It has now been digitised by the New Zealand Electronic Text Centre, also at Victoria.[106]
In popular culture
- A tuatara named "Tua" is prominently featured in the 2017 novel Turtles All The Way Down by John Green.[107]
- The tuatara was the inspiration for a DC Comics superhero, also with a third eye, called Tuatara, member of the Global Guardians.
- There is a brand of New Zealand craft beer named after the Tuatara which particularly references the third eye in its advertising.[108]
- The Tuatara hypercar, designed and manufactured by SSC North America in Tri-Cities, Washington, is named after the reptile, noting its fast evolving DNA and "peaks on the back" as inspiration in the creation of the car.
- The Auckland Tuatara, one of two expansion teams for the 2018/19 Australian Baseball League season, chose the tuatara name to celebrate the resilience of the ancient reptiles, and to raise awareness of New Zealand's commitment to species protection.
- Tuatara Day is 2 May[109] to recognise the day that the tuatara was first recognised not to be a lizard.[9]
See also
References
- "Sphenodon Gray 1831 (rhynchocephalian)". PBDB.
- Hitchmough, R. (2019). "Sphenodon punctatus". IUCN Red List of Threatened Species. 2019: e.T131735762A120191347. Retrieved 14 April 2020.CS1 maint: uses authors parameter (link)
- Australasian Reptile & Amphibian Specialist Group. (1996). "Sphenodon guntheri". IUCN Red List of Threatened Species. 1996: e.T20612A9214663. Retrieved 5 May 2020.CS1 maint: uses authors parameter (link)
- Daugherty C. H.; Cree A.; Hay J. M.; Thompson M. B. (1990). "Neglected taxonomy and continuing extinctions of tuatara (Sphenodon)". Nature. 347 (6289): 177–179. Bibcode:1990Natur.347..177D. doi:10.1038/347177a0. S2CID 4342765.
- Gaze, Peter (2001). Tuatara recovery plan 2001–2011 (PDF). Threatened Species Recovery Plan 47. Biodiversity Recovery Unit, Department of Conservation, Government of New Zealand. ISBN 978-0-478-22131-2. Archived from the original (PDF) on 5 November 2011. Retrieved 2 June 2007.
- Beston, Anne (25 October 2003). "New Zealand Herald: Tuatara Release" (PDF). Archived from the original (PDF) on 4 October 2007. Retrieved 11 September 2007.
- "Tuatara". New Zealand Ecology: Living Fossils. TerraNature Trust. 2004. Archived from the original on 3 May 2017. Retrieved 10 November 2006.
- "The Tuatara". Kiwi Conservation Club: Fact Sheets. Royal Forest and Bird Protection Society of New Zealand Inc. 2009. Archived from the original on 16 October 2015. Retrieved 13 September 2017.CS1 maint: bot: original URL status unknown (link)
- Günther A (1867). "Contribution to the anatomy of Hatteria (Rhynchocephalus, Owen)". Philosophical Transactions of the Royal Society. 157: 595–629. Bibcode:1867RSPT..157..595G. doi:10.1098/rstl.1867.0019. JSTOR 108983.
- Jones ME, Anderson CL, Hipsley CA, Müller J, Evans SE, Schoch RR (2013). "Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara)". BMC Evolutionary Biology. 13 (208): 208. doi:10.1186/1471-2148-13-208. PMC 4016551. PMID 24063680.CS1 maint: multiple names: authors list (link)
- Gemmell, N.J.; Rutherford, K.; Prost, S.; et al. (2020). "The tuatara genome reveals ancient features of amniote evolution". Nature. 584 (7821): 403–409. doi:10.1038/s41586-020-2561-9. PMC 7116210. PMID 32760000.
- "Tuatara". Conservation: Native Species. Threatened Species Unit, Department of Conservation, Government of New Zealand. Archived from the original on 31 January 2011. Retrieved 3 February 2013.
- Rest, Joshua S.; Ast, Jennifer C.; Austin, Christopher C.; Waddell, Peter J.; Tibbetts, Elizabeth A.; Hay, Jennifer M.; Mindell, David P. (1 January 2003). "Molecular systematics of primary reptilian lineages and the tuatara mitochondrial genome". Molecular Phylogenetics and Evolution. 29 (2): 289–297. doi:10.1016/s1055-7903(03)00108-8. PMID 13678684.
- "Reptiles:Tuatara". Animal Bytes. Zoological Society of San Diego. 2007. Archived from the original on 30 November 2012. Retrieved 1 June 2007.
- Johnson, Rebecca N. (5 August 2020). "Tuatara genome reveals diverse insights into a remarkable reptile". Nature. 584 (7821): 351–352. doi:10.1038/d41586-020-02063-4. ISSN 0028-0836.
- Schopf TJ. (1984). "Rates of Evolution and the Notion of" Living Fossils"". Annual Review of Earth and Planetary Sciences. 12: 245–292. doi:10.1146/annurev.ea.12.050184.001333.
- Jones MEH (2008). "Skull shape and feeding strategy in Sphenodon and other Rhynchocephalia (Diapsida: Lepidosauria)". Journal of Morphology. 269 (8): 945–66. doi:10.1002/jmor.10634. PMID 18512698. S2CID 16357353.
- Jones MEH (2009). "Dentary tooth shape in Sphenodon and its fossil relatives (Diapsida: Lepidosauria: Rhynchocephalia)". Front Oral Biol. Frontiers of Oral Biology. 13: 9–15. doi:10.1159/000242382. ISBN 978-3-8055-9229-1. PMID 19828962.
- Jones MEH, Tennyson AJ, Worthy JP, Evans SE, Worthy TH (2009). "A sphenodontine (Rhynchocephalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon)". Proceedings of the Royal Society B. 276 (1660): 1385–90. doi:10.1098/rspb.2008.1785. PMC 2660973. PMID 19203920.CS1 maint: uses authors parameter (link)
- Meloro C, Jones MEH (2012). "Tooth and cranial disparity in the fossil relatives of Sphenodon (Rhynchocephalia) dispute the persistent 'living fossil' label". Journal of Evolutionary Biology. 25 (11): 2194–2209. doi:10.1111/j.1420-9101.2012.02595.x. PMID 22905810. S2CID 32291169.
- Jones MEH, Cree A (2012). "Tuatara". Current Biology. 22 (23): R986–R987. doi:10.1016/j.cub.2012.10.049. PMID 23218010.
- Herrera-Flores, Jorge A.; Stubbs, Thomas L.; Benton, Michael J. (2017). "Macroevolutionary patterns in Rhynchocephalia: is the tuatara (Sphenodon punctatus) a living fossil?". Palaeontology. 60 (3): 319–328. doi:10.1111/pala.12284.
- Vaux, Felix; Morgan-Richards, Mary; Daly, Elizabeth E.; Trewick, Steven A. (2019). "Tuatara and a new morphometric dataset for Rhynchocephalia: Comments on Herrera‐Flores et al". Palaeontology. 62 (2): 321–334. doi:10.1111/pala.12402.
- Herrera-Flores, Jorge A.; Stubbs, Thomas L.; Benton, Michael J. (2019). "Reply to comments on: Macroevolutionary patterns in Rhynchocephalia: is the tuatara (Sphenodon punctatus) a living fossil?" (PDF). Palaeontology. 62 (2): 335–338. doi:10.1111/pala.12404.
- "Tuatara genome mapping". Odt.co.nz. Retrieved 10 June 2018.
- Newman 1987
- Cree, Allison; David Butler (1993). Tuatara Recovery Plan (PDF). Threatened Species Recovery Plan Series No.9. Threatened Species Unit, Department of Conservation, Government of New Zealand. ISBN 978-0-478-01462-4. Archived from the original (PDF) on 30 September 2012. Retrieved 2 June 2007.
- Cree, Alison (2014). Tuatara : biology and conservation of a venerable survivor. Canterbury University Press. ISBN 9781927145449.
- Hay, J.M.; Sarre, S.D.; Lambert, D.M.; Allendorf, F.W.; Daugherty, C.H. (2010). "Genetic diversity and taxonomy: a reassessment of species designation in tuatara (Sphenodon: Reptilia)". Conservation Genetics. 11 (3): 1063–1081. doi:10.1007/s10592-009-9952-7. hdl:10072/30480. S2CID 24965201.
- "Tuatara Factsheet (Sphenodon punctatus)". Sanctuary Wildlife. Karori Sanctuary Wildlife Trust. Archived from the original on 21 October 2007. Retrieved 28 June 2009.
- New Zealand’s ‘living fossil’ confirmed as nesting on the mainland for the first time in 200 years! Archived 27 February 2013 at the Wayback Machine, Karori Sanctuary Trust, 31 October 2008.
- Our first baby tuatara!, Karori Sanctuary Trust, 18 March 2009.
- Cree, Alison. 2002. Tuatara. In: Halliday, Tim and Adler, Kraig (eds.), The new encyclopedia of reptiles and amphibians, Oxford University Press, Oxford, pp. 210–211. ISBN 0-19-852507-9
- "Hylonomus lyelli". Symbols. Province of Nova Scotia. May 2003. Retrieved 24 May 2007.
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, R.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; Hodgson, W.C.; Ignjatovic, V.; Summerhayes, R.; Kochva, E. (2005). "Early evolution of the venom system in lizards and snakes". Nature. 439 (7076): 584–588. Bibcode:2006Natur.439..584F. doi:10.1038/nature04328. PMID 16292255. S2CID 4386245.
- Lutz 2005, p. 42.
- Fraser, Nicholas; Sues, Hans-Dieter; (eds) (1994). "Phylogeny" In the Shadow of the Dinosaurs: Early Mesozoic Tetrapods. Cambridge University Press. ISBN 978-0-521-45242-7.CS1 maint: multiple names: authors list (link) CS1 maint: extra text: authors list (link)
- "Sphenodon". Dictionary.com Unabridged (v 1.1). Random House, Inc. Retrieved 8 January 2007.
- Russell, Matt (August 1998). "Tuatara, Relics of a Lost Age". Cold Blooded News. Colorado Herpetological Society. Archived from the original on 19 April 2012.
- Wu, Xiao-Chun (1994). "Late Triassic-Early Jurassic sphenodontians from China and the phylogeny of the Sphenodontia" in Nicholas Fraser & Hans-Dieter Sues (eds) In the Shadow of the Dinosaurs: Early Mesozoic Tetrapods. Cambridge University Press. ISBN 978-0-521-45242-7.
- "Tuatara evolving faster than any other species". Massey University. 4 January 2008. Retrieved 28 June 2009.
- "Fastest Evolving Creature is 'Living Dinosaur'". LiveScience. 26 March 2008.
- Klein, N.; Scheyer, T.M. (2017). "Microanatomy and life history in Palaeopleurosaurus (Rhynchocephalia: Pleurosauridae) from the Early Jurassic of Germany". The Science of Nature. 104 (4): 4. Bibcode:2017SciNa.104....4K. doi:10.1007/s00114-016-1427-3. PMID 28005148. S2CID 27133670.
- "Tuatara – Sphenodon punctatus". Science and Nature: Animals. bbc.co.uk. Retrieved 28 February 2006.
- Stearn, William T (1 April 2004). Botanical Latin. Portland, Oregon: Timber Press Inc. p. 476. ISBN 978-0-88192-627-9.
- Beolens, Bo; Watkins, Michael; Grayson, Michael (2011). The Eponym Dictionary of Reptiles. Baltimore: Johns Hopkins University Press. xiii + 296 pp. ISBN 978-1-4214-0135-5. (Sphenodon guntheri, pp. 110–111).
- Lutz 2005, p. 16.
- Gill, Brian & Whitaker, Tony. 1996. New Zealand Frogs and reptiles. David Bateman publishing, pp. 22–24. ISBN 1-86953-264-3
- Colenso, W. (1885). "Notes on the Bones of a Species of Sphenodon, (S. diversum, Col.,) apparently distinct from the Species already known" (PDF). Transactions and Proceedings of the Royal Society of New Zealand. 18: 118–128.
- Jacobson, Elliott R. (11 April 2007). Infectious Diseases and Pathology of Reptiles. ISBN 9781420004038.
- "San Diego Zoo's Animal Bytes: Tuatara". San Diego Zoo. Archived from the original on 30 November 2012. Retrieved 19 April 2008.
- "Tuataras at Animal Corner". Archived from the original on 17 March 2015. Retrieved 31 December 2007.
- Whiteside DI (1986). "The head skeleton of the Rhaetian sphenodontid Diphydontosaurus avonis gen. et sp. nov. and the modernizing of a living fossil". Philosophical Transactions of the Royal Society of London B. 312 (1156): 379–430. doi:10.1098/rstb.1986.0014.
- Jones MEH, O'Higgins P, Fagan MJ, Evans SE, Curtis N (2012). "Shearing mechanics and the influence of a flexible symphysis during oral food processing in Sphenodon (Lepidosauria: Rhynchocephalia)". The Anatomical Record. 295 (7): 1075–1091. doi:10.1002/ar.22487. PMID 22644955. S2CID 45065504.CS1 maint: multiple names: authors list (link)
- "A Unique Slice-and-Dice Strategy for Chewing". The New York Times. 5 June 2012.
- Mlot, Christine (8 November 1997). "Return of the Tuatara: A relic from the age of dinosaurs gets a human assist" (PDF). Science News. Science News. Retrieved 24 May 2007.
- Lutz 2005, p. 27.
- Kieser JA, Tkatchenko T, Dean MC, Jones MEH, Duncan W, Nelson NJ. (2009). "Microstructure of dental hard tissues and bone in the tuatara dentary, Sphenodon punctatus (Diapsida: Lepidosauria: Rhynchocephalia)". Comparative Dental Morphology Karger Publishers. Frontiers of Oral Biology. 13: 80–85. doi:10.1159/000242396. ISBN 978-3-8055-9229-1. PMID 19828975.CS1 maint: multiple names: authors list (link)
- Larsson HCE (2001). "Endocranial anatomy of Carcharodontosaurus saharicus (Theropoda: Allosauroidea) and its implications for theropod brain evolution". pp. 19–33. In: Tanke DH, Carpenter K, Skrepnick MW (editors) (2001). Mesozoic Vertebrate Life. Bloomington & Indianapolis: Indiana University Press. 352 pp. ISBN 0-253-33907-3.
- Meyer-Rochow V.B.; Wohlfahrt S.; Ahnelt P.K. (2005). "Photoreceptor cell types in the retina of the tuatara (Sphenodon punctatus) have cone characteristics". Micron. 36 (5): 423–428. doi:10.1016/j.micron.2005.03.009. PMID 15896966.
- Schwab, IR; O'Connor, GR (March 2005). "The lonely eye". Br J Ophthalmol. 89 (3): 256. doi:10.1136/bjo.2004.059105. PMC 1772576. PMID 15751188.
- Halliday, Tim R. 2002. Salamanders and newts: Finding breeding ponds. In: Halliday, Tim and Adler, Kraig (eds.), The new encyclopedia of reptiles and amphibians, Oxford University Press, Oxford, p. 52. ISBN 0-19-852507-9
- Kaplan, Melissa (6 September 2003). "Reptile Hearing". Melissa Kaplan's Herp Care Collection. Retrieved 24 July 2006.
- Romer, A. S. & Parsons, T. S. 1977 The Vertebrate Body. Fifth ed. Philadelphia, W. B Saunders; 624 p.
- "Zoo Berlin: Tuatara". Archived from the original on 14 August 2007. Retrieved 11 September 2007.
- "Tuatara Reptile, New Zealand". Archived from the original on 14 November 2007. Retrieved 31 December 2007.
- Alibardi L.; Meyer-Rochow V.B. (1990). "Ultrastructural survey of the spinal cord of young tuatara (Sphenodon punctatus) with emphasis on the glia". New Zealand Journal of Zoology. 17: 73–85. doi:10.1080/03014223.1990.10422586.
- Alibardi L.; Meyer-Rochow V.B. (1990). "Fine structure of regenerating caudal spinal cord in adult tuatara (Sphenodon punctatus)". Journal für Hirnforschung. 31 (5): 613–621. PMID 1707076.
- Castanet, J.; Newman, D. G.; Girons, H. Saint (1988). "Skeletochronological Data on the Growth, Age, and Population Structure of the Tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand". Herpetologica. 44 (1): 25–37. JSTOR 3892195.
- "Tuatara: Facts". Southland Museum. 18 January 2006. Archived from the original on 9 June 2007. Retrieved 2 June 2007.
- Schofield, Edith (24 March 2009). "New arrivals thrill staff at sanctuary". Otago Daily Times. Retrieved 23 March 2009.
- Musico, Bruce (1999). "Sphenodon punctatus". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 22 April 2006.
- Thompson MB, Daugherty CH (1998). "Metabolism of tuatara, Sphenodon punctatus". Comparative Biochemistry and Physiology A. 119 (2): 519–522. doi:10.1016/S1095-6433(97)00459-5.
- Meyer-Rochow, V.B. (1988). "Behaviour of young tuatara (Sphenodon punctatus) in total darkness". Tuatara. 30: 36–38.
- Meyer-Rochow, V.B.; Teh K.L. "Visual predation by tuatara (Sphenodon punctatus) on the beach beetle (Chaerodes trachyscelides) as a selective force in the production of distinct colour morphs". Tuatara. 31: 1–8.
- Daugherty, Charles, and Keall, Susan. Tuatara: Life History In: Te Ara: The Encyclopedia of New Zealand.
- Lutz 2005, p. 24.
- "Archived copy". Archived from the original on 15 October 2012. Retrieved 31 January 2013.CS1 maint: archived copy as title (link)
- "Reptile's Pet-Store Looks Belie Its Triassic Appeal". The New York Times. 22 November 2010. Retrieved 21 December 2010.
- Cree; Cockrem, J. F.; Guillette, L. J.; et al. (1992). "Reproductive cycles of male and female tuatara (Sphenodon punctatus) on Stephens Island, New Zealand". Journal of Zoology. 226 (2): 199–217. doi:10.1111/j.1469-7998.1992.tb03834.x.
- C. Gand, J.C. Gillingham & D.L. Clark (1984). "Courtship, mating and male combat in Tuatara, Sphenodon punctatus". Journal of Herpetology. 18 (2): 194–197. doi:10.2307/1563749. JSTOR 1563749.
- Lutz 2005, p. 19.
- Brennan, Patricia L.R. (January 2016). "Evolution: One Penis After All". Current Biology. 26 (1): R29–R31. doi:10.1016/j.cub.2015.11.024. ISSN 0960-9822. PMID 26766229.
- Packard M.J.; Hirsch K.F.; Meyer-Rochow V.B. (1982). "Structure of the shell from eggs of the tuatara (Sphenodon punctatus)". Journal of Morphology. 174 (2): 197–205. doi:10.1002/jmor.1051740208. PMID 30096972. S2CID 51957289.
- "Tuatara becomes a father for the first time, aged 111". The New Zealand Herald. APN Holdings. 26 January 2009. Retrieved 28 June 2009.
- "Reptile becomes a father, at 111". BBC News. 26 January 2009. Retrieved 28 June 2009.
- Cree A.; Thompson M. B.; Daugherty C. H. (1995). "Tuatara sex determination". Nature. 375 (6532): 543. Bibcode:1995Natur.375..543C. doi:10.1038/375543a0. S2CID 4339729.
- "110-year-old 'living fossil' becomes a dad". CNN. 30 January 2009. Retrieved 28 June 2009.
- Towns, David R.; Daugherty, Charles H.; Cree, Alison (2001). "Raising the prospects for a forgotten fauna: a review of 10 years of conservation effort for New Zealand reptiles". Biological Conservation. 99: 3–16. doi:10.1016/s0006-3207(00)00184-1. Archived from the original on 24 April 2014. Retrieved 11 March 2012.
- Lutz 2005, pp. 59–60.
- Crook, Ian G. (1973). "The tuatara, Sphenodon punctatus Gray, on islands with and without populations of the Polynesian rat, Rattus exulans (Peale)". Proceedings of the New Zealand Ecological Society. 20: 115–120. JSTOR 24061518.
- Cree A.; Daugherty C.H.; Hay J.M. (1995). "Reproduction of a rare New Zealand reptile, the tuatara Sphenodon punctatus, on rat-free and rat-inhabited islands". Conservation Biology. 9 (2): 373–383. doi:10.1046/j.1523-1739.1995.9020373.x.
- Daugherty, C. H.; Cree, A.; Hay, J. M.; Thompson, M. B. (1 September 1990). "Neglected taxonomy and continuing extinctions of tuatara (Sphenodon)". Nature. 347 (6289): 177–179. Bibcode:1990Natur.347..177D. doi:10.1038/347177a0. ISSN 0028-0836. S2CID 4342765.
- "Rare Reptile Hatchling Found in New Zealand". The Guardian. 20 March 2009.
- Daugherty, Charles and Keall, Susan. Tuatara: Tuatara islands In: Te Ara: The Encyclopedia of New Zealand.
- "Fauna on Little Barrier Island". Department of Conservation. Archived from the original on 29 March 2014. Retrieved 3 February 2013.
- "Rare tuatara raised at Wellington Zoo" (Press release). Wellington Zoo. 29 October 2007. Retrieved 19 April 2008.
- "Reptiles|Tuatara|At the zoo". Retrieved 11 May 2014.
- Translocated reptiles, Part 4, Tiritiri Matangi – An education resource for schools Archived 29 March 2014 at the Wayback Machine. Department of Conservation, November 2007.
- "130 Tuatara Find Sanctuary". The Dominion Post. 20 October 2007. Retrieved 19 April 2008.
- "Life will be wild for new boy". The Dominion Post. 20 March 2009. Retrieved 20 March 2009.
- "Tuatara: Lizard-like reptile takes 38 years to lay an egg in Chester Zoo". The Independent. 31 January 2016. Retrieved 31 January 2016.
- Williams, David (2001). "Chapter 6: Traditional Kaitiakitanga Rights and Responsibilities" (PDF). Wai 262 Report: Matauranga Maori and Taonga. Waitangi Tribunal. Archived from the original (PDF) on 28 June 2007. Retrieved 2 June 2007.
- Ramstad Kristina M.; Nelson N. J.; Paine G.; Beech D.; Paul A.; Paul P.; Allendorf F. W.; Daugherty C. H. (2007). "Species and Cultural Conservation in New Zealand: Maori Traditional Ecological Knowledge of Tuatara". Conservation Biology. 21 (2): 455–464. doi:10.1111/j.1523-1739.2006.00620.x. PMID 17391195.
- Lutz 2005, p. 64.
- "Tuatara : Journal of the Biological Society". New Zealand Electronic Text Centre. Retrieved 19 April 2008.
- "Everything We Know About John Green's New Book". EW.com. 23 June 2017. Retrieved 15 October 2017.
- "About – The Third Eye » Tuatara Breweries". Tuatarabrewing.co.nz. Retrieved 10 June 2018.
- "Tuatara Day". worldwideweirdholidays.com. Retrieved 5 May 2020.
Further reading
- Daugherty, Charles and Cree, Alison. (1990). Tuatara: a survivor from the dinosaur age. New Zealand Geographic 6 (April–June 1990): 60.
- Lutz, Dick (2005). Tuatara: A Living Fossil. Salem, Oregon: DIMI PRESS. ISBN 978-0-931625-43-5.
- McKintyre, Mary (1997). Conservation of the Tuatara. Victoria University Press. ISBN 978-0-86473-303-0.
- Newman, D. G. (1987). Tuatara. Endangered New Zealand Wildlife Series. Dunedin, New Zealand: John McIndoe. ISBN 978-0-86868-098-9.
- Parkinson, Brian (2000). The Tuatara. Reed Children’s Books. ISBN 978-1-86948-831-4.
External links
| Wikimedia Commons has media related to: |
| Wikispecies has information related to Sphenodon. |
- B. Blanchard; et al. (2000). "Tuatara captive management plan and husbandry manual" (PDF). Department of Conservation, Wellington, New Zealand. Retrieved 26 November 2007.
- "Digimorph – Sphenodon punctatus (tuatara) – 3D visualisations from X-ray CT scans". Retrieved 8 May 2006.
- "ARKive – images and movies of the Brothers Island tuatara (Sphenodon guntheri)". Archived from the original on 26 June 2007. Retrieved 3 June 2007.
- "Evolution of a third eye in some animals? MadSci Network". Retrieved 9 August 2007.
- Schwab, IR; O'Connor, GR (2005). "The lonely eye". The British Journal of Ophthalmology. 89 (3): 256. doi:10.1136/bjo.2004.059105. PMC 1772576. PMID 15751188.
- Tuatara in Te Ara – the Encyclopedia of New Zealand
- Specimens of Tuatara in the collection of the Museum of New Zealand Te Papa Tongarewa
- "Tuatara reptile slices food with 'steak-knife teeth'" BBC Nature. Retrieved 8 January 2014.
- Tuatara discussed on RNZ Critter of the Week, 18 August 2017