Vanadium bromoperoxidase
Vanadium bromoperoxidases are a kind of enzymes called haloperoxidases. Its primary function is to remove hydrogen peroxide from in or around the cell which is produced during photosynthesis. By producing hypobromous acid (HOBr) a secondary reaction with dissolved organic matter, what results is the bromination of organic compounds that are associated with the defense of the organism. These enzymes produce the bulk of natural organobromine compounds in the world.
| Bromoperoxidase/chloroperoxidase, Vanadium | |
|---|---|
| Identifiers | |
| Symbol | Br/Cl_peroxidase |
| CATH | 1qhb |
| SCOP2 | 1qhb / SCOPe / SUPFAM |
| CDD | cd03398 |
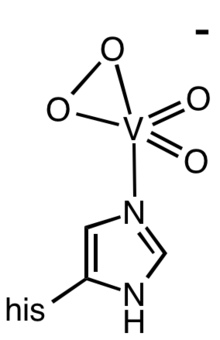
Vanadium bromoperoxidases are one of the few classes of enzymes that requires vanadium. The active site features a vanadium oxide center attached to the protein via one histidine side chain and a collection of hydrogen bonds to the oxide ligands.[1]
Occurrence and function
Vanadium bromoperoxidases have been found in bacteria, fungi, marine macroalgae (seaweeds), and marine microalgea (diatoms) which produce brominated organic compounds.[2] It has not been definitively identified as the bromoperoxidase of higher eukaryotes, such as murex snails, which have a very stable and specific bromoperoxidase, but perhaps not a vanadium dependent one.[3] While the purpose of the bromoperoxidase is still unknown, the leading theories include that it’s a way of regulating hydrogen peroxide produced by photosynthesis and/or as a self-defense mechanism by producing hypobromic acid which prevents the growth of bacteria.[4][5]
The enzymes catalyse the oxidation of bromide (0.0067% of sea water) by hydrogen peroxide. The resulting electrophilic bromonium cation (Br+) attacks hydrocarbons (symbolized as R-H in the following equation):
- R-H + Br− + H2O2 → R-Br + H2O + OH−
The bromination acts on a variety of dissolved organic matter and increasingly bromination leads to the formation of bromoform.[6] The vanadium bromoperoxidases produce an estimated 1–2 million tons of bromoform and 56,000 tons of bromomethane annually.[7] Partially in the polar regions, which has high blooms of microalgae in the spring, these compounds have the potential to enter the troposphere and lower stratosphere.[8][9] Through photolysis, brominated methanes produce a bromine radical (Br−) that can lead to ozone depletion.[10] Most of the earth's natural organobromine compounds arise by the action of this enzyme.
References
- Butler A, Carter-Franklin JN (February 2004). "The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products". Natural Product Reports. 21 (1): 180–8. doi:10.1039/b302337k. PMID 15039842.
- Moore RM, Webb M, Tokarczyk R, Wever R (15 September 1996). "Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures". Journal of Geophysical Research: Oceans. 101 (C9): 20899–20908. Bibcode:1996JGR...10120899M. doi:10.1029/96JC01248.
- Winter JM, Moore BS (July 2009). "Exploring the chemistry and biology of vanadium-dependent haloperoxidases". The Journal of Biological Chemistry. 284 (28): 18577–81. doi:10.1074/jbc.R109.001602. PMC 2707250. PMID 19363038.
- Gribble GW (2009-12-17). Naturally occurring organohalogen compounds : a comprehensive update. Springer-Verlag/Wein. Bibcode:2010nooc.book.....G. ISBN 978-3-211-99322-4.
- Renirie R, Dewilde A, Pierlot C, Wever R, Hober D, Aubry JM (July 2008). "Bactericidal and virucidal activity of the alkalophilic P395D/L241V/T343A mutant of vanadium chloroperoxidase". Journal of Applied Microbiology. 105 (1): 264–70. doi:10.1111/j.1365-2672.2008.03742.x. PMID 18266697.
- Butler A, Sandy M (August 2009). "Mechanistic considerations of halogenating enzymes". Nature. 460 (7257): 848–54. Bibcode:2009Natur.460..848B. doi:10.1038/nature08303. PMID 19675645.
- Gribble GW (1999). "The diversity of naturally occurring organobromine compounds". Chemical Society Reviews. 28 (5): 335–46. doi:10.1039/a900201d.
- Wever R, van der Horst MA (September 2013). "The role of vanadium haloperoxidases in the formation of volatile brominated compounds and their impact on the environment" (PDF). Dalton Transactions. 42 (33): 11778–86. doi:10.1039/c3dt50525a. PMID 23657250.
- Hill VL, Manley SL (May 2009). "Release of reactive bromine and iodine from diatoms and its possible role in halogen transfer in polar and tropical oceans". Limnology and Oceanography. 54 (3): 812–822. Bibcode:2009LimOc..54..812H. doi:10.4319/lo.2009.54.3.0812.
- Saiz-Lopez A, von Glasow R (October 2012). "Reactive halogen chemistry in the troposphere". Chemical Society Reviews. 41 (19): 6448–72. doi:10.1039/c2cs35208g. PMID 22940700.
External links
- active site Interactive image (Jmol)
- Example structure: 1qhb
- Family/Domain classifications:
- SCOP: Family a.111.1.2: Haloperoxidase (bromoperoxidase)
- CATH: Superfamily 1.10.606.10: Vanadium-containing Chloroperoxidase, domain 2
- CDD: cd03398: PAP2_haloperoxidase
- InterPro: whole protein