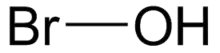
Hypobromous acid
Hypobromous acid is a weak, unstable acid with chemical formula of HOBr. It is mainly produced and handled in an aqueous solution. It is generated both biologically and commercially as a disinfectant. Salts of hypobromite are rarely isolated as solids.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
hypobromous acid, bromic(I) acid, bromanol, hydroxidobromine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.119.006 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HOBr | |
| Molar mass | 96.911 |
| Density | 2.470 g/cm3 |
| Boiling point | 20–25 °C (68–77 °F; 293–298 K) |
| Acidity (pKa) | 8.65 |
| Conjugate base | Hypobromite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and properties
Addition of bromine to water gives hypobromous acid and hydrobromic acid (HBr) via a disproportionation reaction.
- Br2 + H2O HOBr + HBr
In nature, hydrobromous acid is produced by bromoperoxidases, which are enzymes that catalyze the oxidation of bromide with hydrogen peroxide:[1]
- Br− + H2O2 HOBr + OH−
Hypobromous acid has a pKa of 8.65 and is therefore only partially dissociated in water at pH 7. Like the acid, hypobromite salts are unstable and undergo a slow disproportionation reaction to yield the respective bromate and bromide salts.
- 3 BrO−(aq) → 2 Br−(aq) + BrO−
3(aq)
Its chemical and physical properties are similar to those of other hypohalites.
Uses
HOBr is used as a bleach, an oxidizer, a deodorant, and a disinfectant, due to its ability to kill the cells of many pathogens. The compound is generated in warm-blooded vertebrate organisms especially by eosinophils, which produce it by the action of eosinophil peroxidase, an enzyme which preferentially uses bromide.[2] Bromide is also used in hot tubs and spas as a germicidal agent, using the action of an oxidizing agent to generate hypobromite in a similar fashion to the peroxidase in eosinophils. It is especially effective when used in combination with its congener, hypochlorous acid.
References
- Butler, Alison.; Walker, J. V. (1993). "Marine haloperoxidases". Chemical Reviews. 93 (5): 1937–1944. doi:10.1021/cr00021a014.
- Mayeno, AN; Curran, AJ; Roberts, RL; Foote, CS (1989). "Eosinophils preferentially use bromide to generate halogenating agents". The Journal of Biological Chemistry. 264 (10): 5660–8. PMID 2538427. Retrieved 2008-01-12.