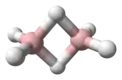Ethynyl radical
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(C—C)) is an organic compound with the chemical formula C≡CH (also written [CCH] or C
2H). It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory.[1] It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope.[2] It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethynyl radical | |||
| Systematic IUPAC name
Ethynyl | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1814004 | |||
| ChEBI | |||
| ChemSpider | |||
| 48916 | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H | |||
| Molar mass | 25.030 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Astronomical Importance
Observations of C2H can yield a large number of insights into the chemical and physical conditions where it is located. First, the relative abundance of ethynyl is an indication of the carbon-richness of its environment (as opposed to oxygen, which provides an important destruction mechanism).[3] Since there are typically insufficient quantities of C2H along a line of sight to make emission or absorption lines optically thick, derived column densities can be relatively accurate (as opposed to more common molecules like CO, NO, and OH). Observations of multiple rotational transitions of C2H can result in estimates of the local density and temperature. Observations of the deuterated molecule, C2D, can test and extend fractionation theories (which explain the enhanced abundance of deuterated molecules in the interstellar medium).[4] One of the important indirect uses for observations of the ethynyl radical is the determination of acetylene abundances.[5] Acetylene (C2H2) does not have a dipole moment, and therefore pure rotational transitions (typically occurring in the microwave region of the spectrum) are too weak to be observable. Since acetylene provides a dominant formation pathway to ethynyl, observations of the product can yield estimates of the unobservable acetylene. Observations of C2H in star-forming regions frequently exhibit shell structures, which implies that it is quickly converted to more complex molecules in the densest regions of a molecular cloud. C2H can therefore be used to study the initial conditions at the onset of massive star formation in dense cores.[6] Finally, high-spectral-resolution observations of Zeeman splitting in C2H can give information about the magnetic fields in dense clouds, which can augment similar observations that are more commonly done in the simpler cyanide (CN).[7]
Formation and destruction
The formation and destruction mechanisms of the ethynyl radical vary widely with its environment. The mechanisms listed below represent the current (as of 2008) understanding, but other formation and destruction pathways may be possible, or even dominant, in certain situations.
Formation
In the laboratory, C2H can be made via photolysis of acetylene (C2H2) or C2HCF3,[8] or in a glow discharge of a mixture of acetylene and helium.[9] In the envelopes of carbon-rich evolved stars, acetylene is created in the thermal equilibrium in the stellar photosphere. Ethynyl is created as a photodissociation product of the acetylene that is ejected (via strong stellar winds) into the outer envelope of these stars. In the cold, dense cores of molecular clouds (prior to star formation) where n > 104 cm−3 and T < 20 K, ethynyl is dominantly formed via an electron recombination with the vinyl radical (C
2H+
3).[10] The neutral-neutral reaction of propynylidyne (C3H) and atomic oxygen also produces ethynyl (and carbon monoxide, CO), though this is typically not a dominant formation mechanism. The dominant creation reactions are listed below.
- C
2H+
3 + e− → C2H + H + H - C
2H+
3 + e− → C2H + H2 - CH3CCH+ + e− → C2H + CH3
- C3H + O → C2H + CO
Destruction
The destruction of ethynyl is dominantly through neutral-neutral reactions with O2 (producing carbon monoxide and formyl, HCO), or with atomic nitrogen (producing atomic hydrogen and C2N). Ion-neutral reactions can also play a role in the destruction of ethynyl, through reactions with HCO+ and H+
3. The dominant destruction reactions are listed below.
- C2H + O2 → HCO + CO
- C2H + N → C2N + H
- C2H + HCO+ → C
2H+
2 + CO - C2H + H+
3 → C
2H+
2 + H2
Method of observation
The ethynyl radical is observed in the microwave portion of the spectrum via pure rotational transitions. In its ground electronic and vibrational state, the nuclei are collinear, and the molecule has a permanent dipole moment estimated to be μ = 0.8 D = 2.7×10−30 C·m.[2] The ground vibrational and electronic (vibronic) state exhibits a simple rigid rotor-type rotational spectrum. However, each rotational state exhibits fine and hyperfine structure, due to the spin-orbit and electron-nucleus interactions, respectively. The ground rotational state is split into two hyperfine states, and the higher rotational states are each split into four hyperfine states. Selection rules prohibit all but six transitions between the ground and the first excited rotational state. Four of the six components were observed by Tucker et al. in 1974,[2] the initial astronomical detection of ethynyl, and 4 years later, all six components were observed, which provided the final piece of evidence confirming the initial identification of the previously unassigned lines.[11] Transitions between two adjacent higher-lying rotational states have 11 hyperfine components. The molecular constants of the ground vibronic state are tabulated below.
Isotopologues
Three isotopologues of the 12C12CH molecule have been observed in the interstellar medium. The change in molecular mass is associated with a shift in the energy levels and therefore the transition frequencies associated with the molecule. The molecular constants of the ground vibronic state, and the approximate transition frequency for the lowest 5 rotational transitions are given for each of the isotopologues in the table below.
Rotational transitions for ethenyl isotopologues Isotopologue Year
discoveredMolecular constants
(MHz)Transition frequencies
(MHz)12C12CH 1974[2] B
D
γ
b
c43674.534
0.1071
−62.606
40.426
12.254N = 1→0
N = 2→1
N = 3→2
N = 4→3
N = 5→487348.64
174694.71
262035.64
349368.85
436691.7912C12CD 1985[4][12] B
D
γ
b
c36068.035
0.0687
−55.84
6.35
1.59N = 1→0
N = 2→1
N = 3→2
N = 4→3
N = 5→472135.80
144269.94
216400.79
288526.69
360646.0013C12CH 1994[13] B
D
γ42077.459
0.09805
−59.84N = 1→0
N = 2→1
N = 3→2
N = 4→3
N = 5→484154.53
168306.70
252454.16
336594.57
420725.5712C13CH 1994[13] B
D
γ42631.3831
0.10131
−61.207N = 1→0
N = 2→1
N = 3→2
N = 4→3
N = 5→485262.36
170522.29
255777.36
341025.13
426263.18
References
- Cochran, E. L.; Adrian, F. J.; Bowers, V. A. (1964). "ESR Study of Ethynyl and Vinyl Free Radicals". Journal of Chemical Physics. 40: 213. Bibcode:1964JChPh..40..213C. doi:10.1063/1.1724865.
- Tucker, K. D.; Kutner, M. L.; Thaddeus, P. (1974). "The Ethynyl Radical C2H – A New Interstellar Molecule". Astrophysical Journal. 193: L115–L119. Bibcode:1974ApJ...193L.115T. doi:10.1086/181646.
- Huggins, P. J.; Carlson, W. J.; Kinney, A. L. (1984). "The distribution and abundance of interstellar C2H". Astronomy & Astrophysics. 133: 347–356. Bibcode:1984A&A...133..347H.
- Vrtilek, J. M.; Gottlieb, C. A.; Langer, W. D.; Thaddeus, P.; Wilson, R. W. (1985). "Laboratory and Astronomical Detection of the Deuterated Ethynyl Radical CCD". Astrophysical Journal. 296: L35–L38. Bibcode:1985ApJ...296L..35V. doi:10.1086/184544.
- Fuente, A.; Cernicharo, J.; Omont, A. (1998). "Inferring acetylene abundances from C2H: the C2H2/HCN abundance ratio". Astronomy & Astrophysics. 330: 232–242. Bibcode:1998A&A...330..232F.
- Beuther, H.; Semenov, D.; Henning, T.; Linz, H. (2008). "Ethynyl (C2H) in Massive Star Formation: Tracing the Initial Conditions?". Astrophysical Journal. 675: L33–L36. arXiv:0801.4493. Bibcode:2008ApJ...675L..33B. doi:10.1086/533412.
- Bel, N.; Leroy, B. (1998). "Zeeman splitting in interstellar molecules. II. The ethynyl radical". Astronomy & Astrophysics. 335: 1025–1028. Bibcode:1998A&A...335.1025B.
- Fahr, A. (2003). "Ultraviolet absorption spectrum and cross-sections of ethynyl (C2H) radicals". Journal of Molecular Spectroscopy. 217: 249. doi:10.1016/S0022-2852(02)00039-5.
- Müller, H. S. P.; Klaus, T.; Winnewisser, G. (2000). "Submillimeter-wave spectrum of the ethynyl radical, CCH, up to 1 THz". Astronomy & Astrophysics. 357: L65. Bibcode:2000A&A...357L..65M.
- Woodall, J.; Agúndez, M.; Markwick-Kemper, A. J.; Millar, T. J. (2007). "The UMIST database for astrochemistry 2006". Astronomy & Astrophysics. 466: 1197. arXiv:1212.6362. Bibcode:2007A&A...466.1197W. doi:10.1051/0004-6361:20064981.
- Tucker, K. D.; Kutner, M. L. (1978). "The Abundance and Distribution of Interstellar C2H". Astrophysical Journal. 222: 859. Bibcode:1978ApJ...222..859T. doi:10.1086/156204.
- Combes, F.; Boulanger, F.; Encrenaz, P. J.; Gerin, M.; Bogey, M.; Demuynck, C.; Destombes, J. L. (1985). "Detection of interstellar CCD". Astronomy & Astrophysics. 147: L25. Bibcode:1985A&A...147L..25C.
- Saleck, A. H.; Simon, R.; Winnewisser, G.; Wouterloot, J. G. A. (1994). "Detection of interstellar 13C12CH and 12C13CH". Canadian Journal of Physics. 72: 747. Bibcode:1994CaJPh..72..747S. doi:10.1139/p94-098.