Wollastonite
Wollastonite is a calcium inosilicate mineral (CaSiO3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or dolomite is subjected to high temperature and pressure, which sometimes occurs in the presence of silica-bearing fluids as in skarns[5] or in contact with metamorphic rocks. Associated minerals include garnets, vesuvianite, diopside, tremolite, epidote, plagioclase feldspar, pyroxene and calcite. It is named after the English chemist and mineralogist William Hyde Wollaston (1766–1828).
| Wollastonite | |
|---|---|
 | |
| General | |
| Category | Inosilicate mineral |
| Formula (repeating unit) | Calcium silicate, CaSiO3 |
| Strunz classification | 9.DG.05 |
| Crystal system | Triclinic Monoclinic polytype exists |
| Crystal class | Pinacoidal (1) (same H-M symbol) |
| Space group | P1 (1A polytype) |
| Unit cell | a = 7.925 Å, b = 7.32 Å, c = 7.065 Å; α = 90.055°, β = 95.217°, γ = 103.42°; Z = 6 |
| Identification | |
| Formula mass | 116.159 g/mol |
| Color | White, colorless or gray |
| Crystal habit | Rare as tabular crystals—commonly massive in lamellar, radiating, compact and fibrous aggregates. |
| Twinning | Common |
| Cleavage | Perfect in two directions at near 90° |
| Fracture | Splintery to uneven |
| Mohs scale hardness | 4.5 to 5.0 |
| Luster | Vitreous or dull to pearly on cleavage surfaces |
| Streak | White |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 2.86–3.09 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.616–1.640 nβ = 1.628–1.650 nγ = 1.631–1.653 |
| Birefringence | δ = 0.015 max |
| 2V angle | Measured: 36° to 60° |
| Melting point | 1540 °C |
| Solubility | Soluble in HCl, insoluble in water |
| Other characteristics | Heat of Formation (@298): -89.61kJ Gibbs Free Energy: 41.78kJ |
| References | [1][2][3][4] |
Some of the properties that make wollastonite so useful are its high brightness and white coloration, low moisture and oil absorption, and low volatile content. Wollastonite is used primarily in ceramics, friction products (brakes and clutches), metalmaking, paint filler, and plastics.
Despite its chemical similarity to the compositional spectrum of the pyroxene group of minerals—where magnesium and iron substitution for calcium ends with diopside and hedenbergite respectively—it is structurally very different, with a third SiO4 tetrahedron[6] in the linked chain (as opposed to two in the pyroxenes).
Production trends

World production data for wollastonite is not available for many countries and those that are available frequently are 2 to 3 years old. Estimated world production of crude wollastonite ore was in the range of 700,000 to 720,000 tonnes in 2016. World reserves of wollastonite were estimated to exceed 100 million tonnes. However, many large deposits have not been surveyed yet.
Large deposits of wollastonite have been identified in China, Finland, India, Mexico, and the United States. Smaller, but significant, deposits have been identified in Canada, Chile, Kenya, Namibia, South Africa, Spain, Sudan, Tajikistan, Turkey, and Uzbekistan.[7]
In 2016, the major producers were China (425,000 tonnes), India (185,000 t), United States (Information withheld for commercial reasons but stated to be in third place), Mexico (67,000 t) and Finland (16,000).[7]
In the United States, wollastonite is mined in Willsboro, New York and Gouverneur, New York. Deposits have also been mined commercially in North Western Mexico.[8]
The price of raw wollastonite varied in 2008 between US$80 and US$500 per tonne depending on the country and size and shape of the powder particles.[8]
Uses
Wollastonite has industrial importance worldwide. It is used in many industries, mostly by tile factories which have incorporated it into the manufacturing of ceramic to improve many performance parameters, and this is due to its fluxing properties, freedom from volatile constituents, whiteness, and acicular particle shape.[9]
In ceramics, wollastonite decreases shrinkage and gas evolution during firing, increases green and fired strength, maintains brightness during firing, permits fast firing, and reduces crazing, cracking, and glaze defects.
Wollastonite is used in a cement announced in 2019 which "reduces the overall carbon footprint in precast concrete by 70%."[10]
In metallurgical applications, wollastonite serves as a flux for welding, a source for calcium oxide, a slag conditioner, and to protect the surface of molten metal during the continuous casting of steel.
As an additive in paint, it improves the durability of the paint film, acts as a pH buffer, improves its resistance to weathering, reduces gloss, reduces pigment consumption, and acts as a flatting and suspending agent.
In plastics, wollastonite improves tensile and flexural strength, reduces resin consumption, and improves thermal and dimensional stability at elevated temperatures. Surface treatments are used to improve the adhesion between the wollastonite and the polymers to which it is added.
As a substitute for asbestos in floor tiles, friction products, insulating board and panels, paint, plastics, and roofing products, wollastonite is resistant to chemical attack, inert, stable at high temperatures, and improves flexural and tensile strength.[8] In some industries, it is used in different percentages of impurities, such as its use as a fabricator of mineral wool insulation, or as an ornamental building material.[11]
Plastics and rubber applications were estimated to account for 25% to 35% of U.S. sales in 2009, followed by ceramics with 20% to 25%; paint, 10% to 15%; metallurgical applications, 10% to 15%; friction products, 10% to 15%; and miscellaneous, 10% to 15%. Ceramic applications probably account for 30% to 40% of wollastonite sales worldwide, followed by polymers (plastics and rubber) with 30% to 35% of sales, and paint with 10% to 15% of sales. The remaining sales were for construction, friction products, and metallurgical applications.
Wollastonite has been studied for carbon mineralization for storage of carbon dioxide. It is among the fastest reacting silicates, but may have high costs associated with carbon storage.[12]
Substitutes

The acicular nature of many wollastonite products allows it to compete with other acicular materials, such as ceramic fiber, glass fiber, steel fiber, and several organic fibers, such as aramid, polyethylene, polypropylene, and polytetrafluoroethylene in products where improvements in dimensional stability, flexural modulus, and heat deflection are sought.
Wollastonite also competes with several nonfibrous minerals or rocks, such as kaolin, mica, and talc, which are added to plastics to increase flexural strength, and such minerals as barite, calcium carbonate, gypsum, and talc, which impart dimensional stability to plastics.
In ceramics, wollastonite competes with carbonates, feldspar, lime, and silica as a source of calcium and silicon. Its use in ceramics depends on the formulation of the ceramic body and the firing method.[7]
Composition
In a pure CaSiO3, each component forms nearly half of the mineral by weight: 48.3% of CaO and 51.7% of SiO2. In some cases, small amounts of iron (Fe), and manganese (Mn), and lesser amounts of magnesium (Mg) substitute for calcium (Ca) in the mineral formula (e.g., rhodonite).[11] Wollastonite can form a series of solid solutions in the system CaSiO3-FeSiO3, or hydrothermal synthesis of phases in the system MnSiO3-CaSiO3.[9]
Geologic occurrence

Wollastonite usually occurs as a common constituent of a thermally metamorphosed impure limestone, it also could occur when the silicon is due to metamorphism in contact altered calcareous sediments, or to contamination in the invading igneous rock. In most of these occurrences it is the result of the following reaction between calcite and silica with the loss of carbon dioxide:[9]
- CaCO3 + SiO2 → CaSiO3 + CO2
Wollastonite may also be produced in a diffusion reaction in skarn, it develops when limestone within a sandstone is metamorphosed by a dike, which results in the formation of wollastonite in the sandstone as a result of outward migration of Ca.[9]
Structure
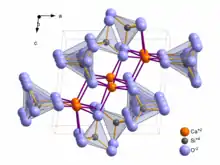
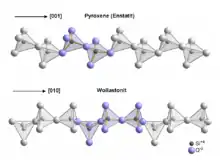
Wollastonite crystallizes triclinically in space group P1 with the lattice constants a = 7.94 Å, b = 7.32 Å, c = 7.07 Å; α = 90,03°, β = 95,37°, γ = 103,43° and six formula units per unit cell.[13] Wollastonite was once classed structurally among the pyroxene group, because both of these groups have a ratio of Si:O = 1:3. In 1931, Warren and Biscoe showed that the crystal structure of wollastonite differs from minerals of the pyroxene group, and they classified this mineral within a group known as the pyroxenoids.[9] It has been shown that the pyroxenoid chains are more kinked than those of pyroxene group, and exhibit longer repeat distance. The structure of wollastonite contains infinite chains of [SiO4] tetrahedra sharing common vertices, running parallel to the b-axis. The chain motif in wollastonite repeats after three tetrahedra, whereas in pyroxenes only two are needed. The repeat distance in the wollastonite chains is 7.32 Å and equals the length of the crystallographic b-axis.
Molten CaSiO3, maintains a tetrahedral SiO4 local structure, at temperatures up to 2000 ˚C.[14] The nearest neighbour Ca-O coordination decreases from 6.0(2) in the room temperature glass to 5.0(2) in the 1700 ˚C liquid, coincident with an increasing number of longer Ca-O neighbors.[15][16]
Physical and optical properties
Wollastonite occurs as bladed crystal masses, single crystals can show an acicular particle shape and usually it exhibits a white color, but sometimes cream, grey or very pale green.
The streak of wollastonite is white, its Mohs hardness is 4.5–5 and specific gravity is 2.87–3.09. There are more than one cleavage planes for it, there is a perfect cleavage on {100}, good cleavages on {001}, and {102}, and an imperfect cleavage on {101}. It is common for wollastonite to have a twin axis [010], a composition plane (100), and rarely to have a twin axis [001]. The luster is usually vitreous to pearly. The melting point of wollastonite is about 1540 ˚C.
See also
- Enstatite – Pyroxene: magnesium-iron silicate with MgSiO3 and FeSiO3 end-members
- Rhodonite – Single chain manganese inosilicate (MnSiO3)
- List of minerals
- List of minerals named after people
References
![]() This article incorporates public domain material from the United States Geological Survey document: "Wollastonite".
This article incorporates public domain material from the United States Geological Survey document: "Wollastonite".
- Wollastonite, Mindat
- Wollastonite, Webmineral
- Wollastonite, Handbook of Mineralogy
- American Mineralogist, V. 79, pp. 134-144, 1994
- Whitley, Sean; Halama, Ralf; Gertisser, Ralf; Preece, Katie; Deegan, Frances M.; Troll, Valentin R. (2020-10-18). "Magmatic and Metasomatic Effects of Magma–Carbonate Interaction Recorded in Calc-silicate Xenoliths from Merapi Volcano (Indonesia)". Journal of Petrology. 61 (4). doi:10.1093/petrology/egaa048. ISSN 0022-3530.
- William Alexander Deer; Robert Andrew Howie; J. Zussman (1992). An introduction to the rock-forming minerals. Longman Scientific & Technical. ISBN 978-0-470-21809-9.
- Wollastonite, USGS Mineral Commodity Summaries 2017
- Robert L. Virta Wollastonite, USGS 2009 Minerals Yearbook (October 2010)
- Deer, Howie and Zussman. Rock Forming Minerals; Single Chain Silicates, Vol. 2A, Second Edition, London, The Geological Society, 1997.
- Alter, Lloyd (August 15, 2019). "LafargeHolcim is selling CO2-sucking cement for precast, reduces emissions by 70 percent". TreeHugger. Retrieved 2019-08-17.
- Andrews, R. W. Wollastonite. London, Her Majesty's Stationery Office, 1970.
- National Academies of Sciences, Engineering, and Medicine (2019). "Chapter 6, Carbon mineralization of CO2". Negative Emissions Technologies and Reliable Sequestration: A Research Agenda (Report). Washington, DC: The National Academies Press. doi:10.17226/25259.CS1 maint: multiple names: authors list (link)
- Buerger, M. J. (1961). "The crystal structures of wollastonite and pectolite". Proceedings of the National Academy of Sciences. 47 (12): 1884–1888. Bibcode:1961PNAS...47.1884B. doi:10.1073/pnas.47.12.1884. JSTOR 71064. PMC 223235. PMID 16578516.
- Benmore, C.J.; et al. (2010). "Temperature-dependent structural heterogeneity in calcium silicate liquids". Phys. Rev. B. 82 (22): 224202. Bibcode:2010PhRvB..82v4202B. doi:10.1103/PhysRevB.82.224202.
- Skinner, L.B.; et al. (2012). "Structure of Molten CaSiO3: Neutron Diffraction Isotope Substitution with Aerodynamic Levitation and Molecular Dynamics Study". J. Phys. Chem. B. 116 (45): 13439–13447. doi:10.1021/jp3066019. PMID 23106223.
- Eckersley, M.C.; et al. (1988). "Structural ordering in a calcium silicate glass". Nature. 355 (6190): 525–527. Bibcode:1988Natur.335..525E. doi:10.1038/335525a0. S2CID 4360261.