5-Methyluridine
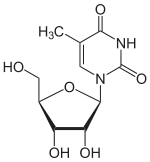
The chemical compound 5-methyluridine, also called ribothymidine, is a pyrimidine nucleoside. Abbreviated m5U, it is the ribonucleoside counterpart to the deoxyribonucleoside thymidine, which lacks a hydroxyl group at the 2' position. 5-Methyluridine contains a thymine base joined to a ribose pentose sugar.[3] It is a white solid.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione | |
| Other names
Ribothymidine, Ribosylthymine; Thymine riboside, m5U | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.522 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14N2O6 | |
| Molar mass | 258.23 g/mol |
| Density | 1,6 g/cm3[1] |
| Melting point | 184[2] °C (363 °F; 457 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
References
- "5-Methyluridine". ChemSpider. Retrieved December 6, 2018.
- William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 3-400. ISBN 978-1-4987-5429-3.
- Shobbir Hussain (2019). "Catalytic crosslinking-based methods for enzyme-specified profiling of RNAribonucleotide modifications". Methods. 156: 60-65. doi:10.1016/j.ymeth.2018.10.003.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.