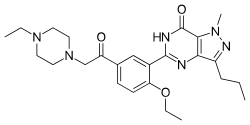
Acetildenafil
Acetildenafil (hongdenafil) is a synthetic drug which acts as a phosphodiesterase inhibitor.[1] It is an analog of sildenafil (Viagra)[2] which has been detected in numerous different brands of supposedly "herbal" aphrodisiac products sold to boost libido and alleviate erectile dysfunction.[3]
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a699015 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H34N6O3 |
| Molar mass | 466.586 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
A range of designer analogs of licensed PDE5 inhibitors such as sildenafil and vardenafil have been detected in recent years in over-the-counter herbal aphrodisiac products,[4][5][6][7] in an apparent attempt to circumvent both the legal restrictions on sale of erectile dysfunction drugs, which are prescription-only medicines in most Western countries, and the patent protection which allows sale of these drugs by competitors only with permission from the patent holders (typically, under a license from the inventors) and to introduce efficacy into otherwise ineffective herbal products. These compounds have been demonstrated to display PDE5 inhibitory activity in vitro and presumably have similar effects when consumed, but have undergone no formal testing in either humans or animals, and as such may represent significant health risks to consumers of these products due to their unknown safety profile.[3][8] Some attempts have been made to ban these drugs as unlicensed medicines, but progress has been slow so far, as even in those jurisdictions which have laws targeting designer drugs, the laws are drafted to ban analogs of illegal drugs of abuse, rather than analogs of prescription medicines. However, at least one court case has resulted in a product being taken off the market.[9]
See also
References
- "Acetildenafil". Cayman Chemical. Retrieved 26 Mar 2017.
- Blok-Tip L, Zomer B, Bakker F, Hartog KD, Hamzink M, Ten Hove J, et al. (August 2004). "Structure elucidation of sildenafil analogues in herbal products". Food Additives and Contaminants. 21 (8): 737–48. doi:10.1080/02652030412331272467. hdl:10029/11381. PMID 15370823.
- Poon WT, Lam YH, Lai CK, Chan AY, Mak TW (October 2007). "Analogues of erectile dysfunction drugs: an under-recognised threat". Hong Kong Medical Journal = Xianggang Yi Xue Za Zhi. 13 (5): 359–63. PMID 17914141.
- Venhuis BJ, de Kaste D (October 2012). "Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: a history, analytical aspects and health risks". Journal of Pharmaceutical and Biomedical Analysis. 69: 196–208. doi:10.1016/j.jpba.2012.02.014. PMID 22464558.
- Zou P, Oh SS, Hou P, Low MY, Koh HL (February 2006). "Simultaneous determination of synthetic phosphodiesterase-5 inhibitors found in a dietary supplement and pre-mixed bulk powders for dietary supplements using high-performance liquid chromatography with diode array detection and liquid chromatography-electrospray ionization tandem mass spectrometry". Journal of Chromatography A. 1104 (1–2): 113–22. doi:10.1016/j.chroma.2005.11.103. PMID 16364350.
- Gratz SR, Gamble BM, Flurer RA (2006). "Accurate mass measurement using Fourier transform ion cyclotron resonance mass spectrometry for structure elucidation of designer drug analogs of tadalafil, vardenafil and sildenafil in herbal and pharmaceutical matrices". Rapid Communications in Mass Spectrometry. 20 (15): 2317–27. Bibcode:2006RCMS...20.2317G. doi:10.1002/rcm.2594. PMID 16817245.
- Hou P, Zou P, Low MY, Chan E, Koh HL (September 2006). "Structural identification of a new acetildenafil analogue from pre-mixed bulk powder intended as a dietary supplement". Food Additives and Contaminants. 23 (9): 870–5. doi:10.1080/02652030600803856. PMID 16901855.
- Oh SS, Zou P, Low MY, Koh HL (November 2006). "Detection of sildenafil analogues in herbal products for erectile dysfunction". Journal of Toxicology and Environmental Health. Part A. 69 (21): 1951–8. doi:10.1080/15287390600751355. PMID 16982533.
- Venhuis BJ, Blok-Tip L, de Kaste D (May 2008). "Designer drugs in herbal aphrodisiacs". Forensic Science International. 177 (2–3): e25-7. doi:10.1016/j.forsciint.2007.11.007. PMID 18178354.