Mesembrine
Mesembrine is an alkaloid present in Sceletium tortuosum (kanna).[1] It has been shown to act as a serotonin reuptake inhibitor (Ki = 1.4 nM), and more recently, has also been found to behave as a weak inhibitor of the enzyme phosphodiesterase 4 (PDE4) (Ki = 7,800 nM).[2] As such, mesembrine may contribute to the antidepressant effects of kanna.[3] The levorotatory isomer, (−)-mesembrine, is the natural form.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3aS,7aS)-3a-(3,4-Dimethoxyphenyl)-1-methyl-2,3,4,5,7,7a-hexahydroindol-6-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H23NO3 | |
| Molar mass | 289.375 g·mol−1 |
| log P | 1.1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rat studies have evaluated effects of kanna extract, finding analgesic and antidepressant potential.[5] No adverse results were noted for a commercial extract up to 5000 mg/kg daily.[6]
Total synthesis
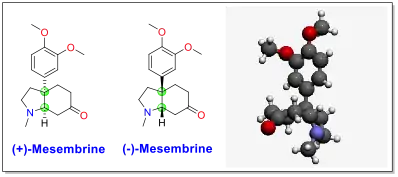
Mesembrine was first isolated and characterized by Bodendorf, et al. in 1957.[7] It is a tricyclic molecule and has two bridgehead chiral carbons between the five-membered ring and the six-membered ring (highlighted in green in the figure below). Because of its structure and bioactivity, mesembrine has been a target for total synthesis over the past 40 years. Over 40 total syntheses have been reported for mesembrine, most of which focused on different approaches and strategies for the construction of the bicyclic ring system and the quaternary carbon.
The first total synthesis of mesembrine was reported by Shamma, et al.[8] in 1965. This route has 21 steps, which was among the longest synthetic routes for mesembrine. Key steps involve the construction of the six-membered ketone ring by Diels-Alder reaction, α-allylation for synthesis of the quaternary carbon, and conjugate addition reaction for the final five-membered ring closure. The final product from this route is a racemic mixture of (+)- and (-)-mesembrine.
-Mesembrine.png.webp)
In 1971, Yamada, et al.[9] reported the first asymmetric total synthesis of (+)-mesembrine. The quaternary carbon was introduced by asymmetric Robinson annulation reaction mediated by an L-proline derivative.
-Mesembrine..png.webp)
References
- Smith, M. T.; Crouch, N. R.; Gericke, N.; Hirst, M. (March 1996). "Psychoactive constituents of the genus Sceletium N.E.Br. and other Mesembryanthemaceae: A Review". Journal of Ethnopharmacology. 50 (3): 119–130. doi:10.1016/0378-8741(95)01342-3. PMID 8691846.
- Harvey, A. L.; Young, L. C.; Viljoen, A. M.; Gericke, N. P. (October 2011). "Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids". Journal of Ethnopharmacology. 137 (3): 1124–1129. doi:10.1016/j.jep.2011.07.035. PMID 21798331.
- Stafford, G. I.; Pedersen, M. E.; van Staden, J.; Jäger, A. K. (October 2008). "Review on plants with CNS-effects used in traditional South African medicine against mental diseases". Journal of Ethnopharmacology. 119 (3): 513–537. doi:10.1016/j.jep.2008.08.010. PMID 18775771.
- Coggon, P.; Farrier, D.S.; Jeffs, P.W.; McPhail, A.T. (1970). "Absolute configuration of mesembrine and related alkaloids: X-ray analysis of 6-epimesembranol methiodide". J. Chem. Soc. B: 1267–1271. doi:10.1039/J29700001267.
- Loria, M. J.; Ali, Z; Abe, N; Sufka, K. J.; Khan, I. A. (Aug 8, 2014). "Effects of Sceletium tortuosum in rats". J. Ethnopharmacol. 155 (1): 731–5. doi:10.1016/j.jep.2014.06.007. PMID 24930358.
- Murbach, T. S.; Hirka, G; Szakonyiné, I. P.; Gericke, N; Endres, J. R. (Dec 2014). "A toxicological safety assessment of a standardized extract of Sceletium tortuosum (Zembrin(®)) in rats". Food Chem. Toxicol. 74: 190–9. doi:10.1016/j.fct.2014.09.017. PMID 25301237.
- Bodendorf, K.; Krieger, W., Arch. Pharm. 1957, 290, 441
- Shamma, M.; Rodriguez, H., Tetrahedron Lett. 1965, 6, 4847. doi:10.1016/S0040-4039(01)89046-8
- Yamada, S; Otani, G. Tetrahedron Lett. 1971, 12, 1133.