Iridium(IV) oxide
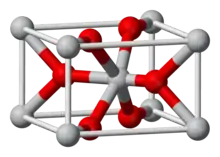
Iridium(IV) oxide, IrO2, is the only well characterised oxide of iridium. It is a blue black solid. The compound adopts the TiO2, rutile structure, featuring six coordinate iridium and three coordinate oxygen.[1]
 | |
| Names | |
|---|---|
| Other names
Iridium dioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.572 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| IrO2 | |
| Molar mass | 224.22 g/mol |
| Appearance | blue-black solid |
| Density | 11.66 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) decomposes |
| insoluble | |
| +224.0·10−6 cm3/mol | |
| Structure | |
| Rutile (tetragonal) | |
| Octahedral (Ir); Trigonal (O) | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
iridium(IV) fluoride, iridium disulfide |
Other cations |
rhodium dioxide, osmium dioxide, platinum dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is used with other rare oxides in the coating of anode-electrodes for industrial electrolysis and in microelectrodes for electrophysiology research.[2]
As described by its discoverers, it can be formed by treating the green form of iridium trichloride with oxygen at high temperatures:
- IrCl3 + O2 → IrO2 + 1.5 Cl2
A hydrated form is also known.[3]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Cogan, Stuart F. (August 2008). "Neural Stimulation and Recording Electrodes". Annual Review of Biomedical Engineering. 10 (1): 275–309. doi:10.1146/annurev.bioeng.10.061807.160518. PMID 18429704.
- H. L. Grube (1963). "The Platinum Metals". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1590.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.