FGFR1OP2
Fibroblast growth factor receptor oncogene partner 2 (FGFR1OP2) was identified in a study on myeloproliferative syndrome (EMS). The study aimed to identify the partner genes to the fibroblast growth factor receptor 1 (FGFR1) involved in the syndrome. Using the 5'-RACE PCR technique, FGFR1OP2 was identified as a novel gene with no known function.[1]
Function
FGFR1OP2, when fused with the fibroblast growth factor receptor 1 (FGFR1), is shown to cause myeloproliferative syndrome.[1] The protein encoded by the FGFR1 gene belongs to the fibroblast growth factor receptor family.[2] FGFRs usually contain an extracellular ligand binding domain, a single transmembrane domain, and an intracellular tyrosine kinase domain. The extracellular domain specifies which ligand the receptor will bind to and mediates ligand-induced receptor dimerization.[3] When FGFR1OP2 is fused to FGFR1, it may exhibit constitutive kinase activity.[4] Furthermore, FGFR1OP2 is possibly involved in some steps of the wound healing pathway.[5]
Evolutionary Biology
The following tables compare the Homo sapiens FGFR1OP2 gene and protein to orthologs. In both of the following tables, the divergence from the Homo sapiens FGFR1OP2 gene or protein to the ortholog was found using TimeTree.[6] Ortholog mRNA and protein sequences were found using NCBI's BLAST [7] and UCSC's BLAT Tool.[8] The accession numbers, as well as the sequence length and the sequence similarity were compiled using BLAST.[7]
| Genus species | Common name | Divergence (MYA) | Accession number | Sequence length (base pairs) | Sequence similarity |
| Homo sapiens | Human | 0 | NP_056448.1 | 3030 | 100% |
| Nomascus leucogenys | Gibbon | 20.4 | XM_003265627.1 | 3020 | 96% |
| Bos taurus | Cow | 94.2 | BC148973.1 | 2616 | 94% |
| Canis lupus familiaris | Dog | 94.2 | NM_001197313.1 | 694 | 94% |
| Loxodonta africana | Elephant | 98.7 | XM_003405700.1 | 762 | 93% |
| Sciurus vulgaris | Squirrel | 92.3 | NA | 1859 | 92% |
| Mus musculus | Mouse | 92.3 | NM_026218.2 | 2828 | 89% |
| Rattus norvegicus | Rat | 92.3 | NM_201421.1 | 2860 | 88% |
| Monodelphis domestica | Opossum | 162.6 | XM_001362357.1 | 765 | 88% |
| Taeniopygia guttata | Zebra finch | 296 | XM_002194575.2 | 1071 | 85% |
| Gallus gallus | Chicken | 296 | NM_001007855.1 | 3142 | 83% |
| Meleagris gallopavo | Turkey | 296 | XM_003202514.1 | 1275 | 82% |
| Anolis carolinensis | Anole | 296 | XM_003221530.1 | 1964 | 82% |
| Trichechus inunguis | Manatee | 98.7 | NA | 2752 | 81% |
| Oreochromis niloticus | Tilapia | 400.1 | XM_003455706.1 | 937 | 79% |
| Xenopus laevis | Frog | 371.2 | NM_001085932.1 | 1279 | 79% |
| Danio rerio | Zebrafish | 400.1 | NM_199955 | 1501 | 78% |
The mRNA orthologs sequence similarity to Homo sapiens FGFR1OP2 was graphed as a function of time in order to show how the FGFR1OP2 gene has changed over time. The graph is depicted on the right.
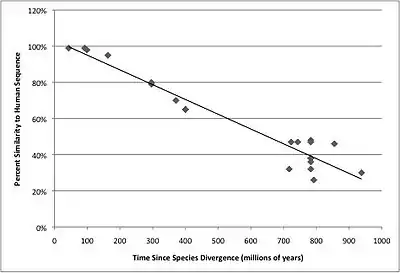
The table below shows the protein orthologs to the Homo sapiens FGFR1OP2 protein. FGFR1OP2 is conserved in all clades of the animal kingdom, as seen in the table below.
| Genus species | Common name | Divergence (MYA) | Accession number | Sequence length (amino acids) | Sequence similarity |
| Homo sapiens | Human | 0 | NP_056448.1 | 253 | 100% |
| Saimiri boliviensis boliviensis | Squirrel monkey | 42.6 | XP_003926645.1 | 253 | 99% |
| Loxodonta africana | Elephant | 98.7 | XP_003405748.1 | 253 | 99% |
| Mus musculus | Mouse | 92.3 | NP_080494.1 | 253 | 99% |
| Monodelphis domestica | Opossum | 162.6 | XP_001362394.1 | 254 | 96% |
| Meleagris gallopavo | Turkey | 296 | XP_003202562.1 | 215 | 83% |
| Anolis carolinensis | Anole | 296 | XP_003221578.1 | 214 | 82% |
| Oreochromis niloticus | Tilapia | 400.1 | XP_003455754.1 | 224 | 78% |
| Xenopus laevis | Frog | 371.2 | NP_001079401.1 | 215 | 77% |
| Danio rerio | Zebrafish | 400.1 | NP_956249.1 | 215 | 77% |
| Strongylocentrotus purpuratus | Sea urchin | 742.9 | XP_786805.2 | 250 | 66% |
| Crassostrea gigas | Oyster | 782.7 | EKC25301.1 | 233 | 64% |
| Capitella teleta | Annelid | 782.7 | ELU02494.1 | 287 | 63% |
| Nematostella vectensis | Sea anemone | 855.3 | XP_001639733.1 | 174 | 62% |
| Ciona intestinalis | Sea squirt | 722.5 | XP_002130340.1 | 236 | 61% |
| Tribolium castaneum | Beetle | 782.7 | XP_974301.1 | 201 | 57% |
| Loa loa | Nematode | 937.5 | EFO20048.2 | 266 | 51% |
| Schistosoma mansoni | Blood fluke | 792.4 | CCD58880.1 | 342 | 51% |
| Amphimedon queenslandica | Sponge | 716.5 | XP_003387498.1 | 221 | 48% |
Gene
There are three transcript variants for the FGFR1OP2 gene, with the first being the longest.[9] FGFR1OP2 is also known as HSPC123-like protein (HSPC123L) and wound inducible transcript 3.0 (wit3.0).[9]
Locus
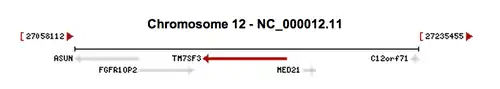
The Homo sapiens FGFR1OP2 gene is located on chromosome 12, with its specific locus being 12p11.23.[9] The Homo sapiens asunder spermatogenesis regulator (ASUN) gene (NCBI Reference Sequence NM_018164.2) is located directly upstream from FGFR1OP2.[11] The ASUN gene is a regulator of development and the mitotic cell cycle.[12] The Homo sapiens transmembrane 7 superfamily member 3 (TM7SF3) gene is located slightly downstream from FGFR1OP2.[13]
Promoter
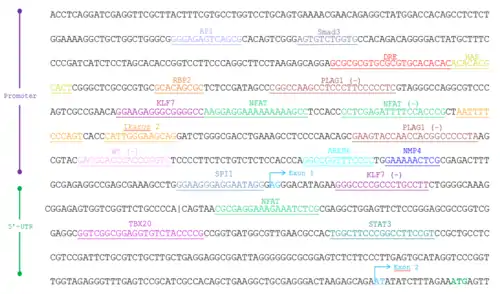
| Transcription factor (T.F.) | Full name | Function | Matrix similarity | Strand T.F. binds | Sequence T.F. binds |
| AP1 | Activator protein 1 | Differentiation, proliferation, apoptosis | 0.874 | + | gggaGAGTcagcg |
| Smad3 | Mothers against decapentaplegic homolog 3 | TGF-beta signaling factor | 0.983 | + | agtGTCTggtg |
| DRE | Dioxin response element | Bound by AHR/AHRNT heterodimer | 0.971 | + | gcgcgcgtgcGCGTgcacacacaca |
| HAS | HIF-1 ancillary sequence | Induce vascular endothelial growth | 0.923 | + | acaCACGcact |
| RBP2 | Retinoblastoma-binding protein 2 | Demethylase | 1.000 | + | GCACagcgc |
| PLAG1 | Pleomorphic adenoma gene 1 | Cell proliferation | 1.000 | - | gaGGGGgaagggaggcttggccg |
| KLF7 | Kruppel-like factor 7 | Regulate cell proliferation, differentiation, and survival | 0.972 | + | ggaagagGGCGgggcca |
| NFAT | Nuclear factor of activated T-cells | Immune response | 0.994 | + | aaggaGGAAaaaaaaagcc |
| NFAT | Nuclear factor of activated T-cells | Immune response | 0.955 | - | cgggtGGAAaatctcgagg |
| Ikaros2 | Ikaros zinc finger | Potential regulator of lymphocytes | 0.986 | + | cattGGGAagcag |
| Ikaros2 | Ikaros zinc finger | Potential regulator of lymphocytes | 0.980 | - | gactGGGAaaatt |
| PLAG1 | Pleomorphic adenoma gene 1 | Cell proliferation | 1.000 | - | taGGGGgccgtggttggtacttc |
| WT | Wilms tumor suppressor | EGR/nerve growth factor | 0.948 | - | gaccgggTGGGtgggtc |
| AREB6 | Atp1a1 regulatory element binding factor 6 | Negative regulator of IL-2 | 0.982 | + | ggccgGTTTcccc |
| NMP4 | Nuclear matrix protein 4 | Cas-interacting zinc finger protein | 0.994 | + | ggAAAAactcg |
| SPI1 | SPI-1 proto-oncogene | Hematopoietic transcription factor | 0.918 | + | ggaagggaGGAAtagg |
| KLF7 | Kruppel-like factor 7 | Regulate cell proliferation, differentiation, and survival | 0.962 | - | aaggcagGGCGgggccc |
| NFAT | Nuclear factor of activated T-cells | Immune response | 0.989 | + | cgcgaGGAAagaaatctcg |
| TBX20 | Brachyury gene | Mesoderm developmental factor | 1.000 | + | ggtcggcggAGGTgtctaccccg |
| STAT3 | Signal transducer and activator of transcription 3 | Activate transcription | 0.940 | + | tggcTTCCcggccttccgt |
Protein

There are three isoforms of the FGFR1OP2 protein. Transcript variant 1 consists of 253 amino acids and weighs 29.4 kilodaltons.[9] FGFR1OP2's isoelectric point is 5.61.[14] The FGFR1OP2 protein does not have a signal sequences, and therefore is not secreted.[15]
Domains
FGFR1OP2 has a domain of unknown function, designated DUF837.[9]
Protein Structure
Using the PELE program of Biology WorkBench the protein sequence of FGFR1OP2 was analyzed, and FGFR1OP2 appears to be completely composed of alpha helices.[14] No structural models for the Homo sapiens FGFR1OP2 protein could be found, but the Mus musculus FGFR1OP2 protein's structure can be seen below.
Expression
The expression of FGFR1OP2 was analyzed via the Gene Expression Omnibus at NCBI.[16] The following are findings from the Gene Expression Onmibus database:
- There is a slightly elevated expression level of FGFR1OP2 in pulmonary sarcoidosis, suggesting FGFR1OP2 operates in part of the wound healing pathway.
- FGFR1OP2 is strongly upregulated when compared to the control in an increased immune response triggered by the VAF347 ligand. FGFR1OP2 is upregulated in the monocyte derived dendritic cell response to the VAF347 ligand. VAF347 activates the aryl hydrocarbon receptor and acts on monocytes and naive CD4+ Th cells to promote development of IL-22 secreting Th cells.[17]
- Langerhans cells show decreased expression of FGFR1OP2 with the null aryl hydrocarbon receptor (ligand is VAF347) in Mus musculus.
- The gene is also highly expressed compared to control samples in monocytopenia.
- It is expressed in cases of leukemia; it may have a linkage to the disease.
- FGFR1OP2 shows low expression levels in septic splenocytes in Mus musculus.
- FGFR1OP2 is expressed in fetal reticulocytes but not adult reticulocytes, suggesting it may play a role in the development of red blood cells.
| FGFR1OP2 GEO Profiles[16] | |||
|---|---|---|---|
| Condition or cell | GEO Profile | Condition or cell | GEO Profile |
| Pulmonary sarcoidosis |  There is a slightly elevated expression level of FGFR1OP2 in pulmonary sarcoidosis. | Monocyte-derived dendritic cell response to VAF347 ligand | |
| Langerhan cells | 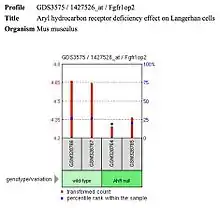 FGFR1OP2 shows decreased expression in Langerhan cells in Mus musculus. | Autosomal dominant monocytopenia |  FGFR1OP2 expression in autosomal dominant monocytopenia. |
| Septic splenocytes | 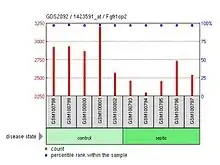 FGFR1OP2 shows low expression in septic splenocytes | Fetal and adult reticulocytes |  FGFR1OP2 expression differs among fetal and adult reticulocytes. |
Interactions
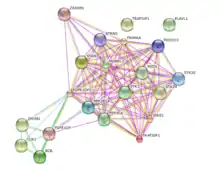
Using the STRING database and Gene Cards, proteins that possibly interact with FGFR1OP2 were identified, and they are shown in the table below.[5][18]
| Interactant | Full name | Function | Source(s) |
| STK24 | Serine/threonine kinase 24 | Protein kinase | Gene Cards |
| TRAF3IP3 | TRAF3 interacting protein | Adapter molecule | Gene Cards, STRING |
| ZRANB1 | Zinc finger, RAN-binding domain containing 1 | Positive regulator of Wnt signaling, cytoskeletal organization | Gene Cards |
| PPP2R1A | Protein phosphatase 2 | Negative control of cell growth and division | Gene Cards |
| STRN | Striatin, calmodulin binding protein | Scaffold protein | Gene Cards, STRING |
| FAM40A | Family with sequence similarity 40, member A | Cytoskeletal organization | STRING |
| PDCD10 | Programmed cell death 10 | Regulate apoptotic pathways | STRING |
| MST4 | Serine/threonine kinase 3 | Mediator of cell growth | STRING |
| SIKE1 | Suppressor of IKBKE1 | Suppressor of IKK-epsilon and TBK1 inhibitor | STRING |
| MOBKL3 | Mps one binder kinase activator-like 3 | Spindle pole body duplication and mitotic checkpoint regulation | STRING |
Clinical Significance
Single-nucleotide polymorphisms (SNPs) in the FGFR1OP2 gene were found to lead to edentulism in the mandible of a small Korean population (134 subjects aged 60–80 years).[19] Also, when FGFR1OP2 is fused to FGFR1, 8p11 myeloproliferative syndrome can result.[1]
References
- Grand, E. K. (2006). "Identification of a novel gene, fgfr1op2, fused to fgfr1 in 8p11 myeloproliferative syndrome". Genes, Chromosomes & Cancer. 40 (1): 78–83. doi:10.1002/gcc.20023. PMID 15034873. S2CID 511788.
- Ornitz, DM; Xu (1996). "Receptor specificity of the fibroblast growth factor family". Journal of Biological Chemistry. 271 (25): 15292–15297. doi:10.1074/jbc.271.25.15292. PMID 8663044.
- J. Schlessinger, A. Ullrich (September 1992). "Growth factor signaling by receptor tyrosine kinases". Neuron. 9 (3): 383–391. doi:10.1016/0896-6273(92)90177-f. PMID 1326293. S2CID 5515795.
- "FGFR1OP2". PhosphoSitePlus®. Retrieved 2013-01-27.
- "FGFR1OP2". GeneCards. Retrieved 2013-01-27.
- Hedges SB, Dudley J & Kumar S. "TimeTree: a public knowledge-base of divergence times among organisms". Retrieved 12 February 2013.
- "BLAST (Basic Local Alignment Search Tool)". NCBI. Retrieved 3 May 2013.
- Kent, Jim. "BLAT". UCSC Genome Bioinformatics. Retrieved 27 March 2013.
- "Homo sapiens FGFR1 oncogene partner 2 (FGFR1OP2), transcript variant 1, mRNA".
- "ElDorado". Genomatix. Retrieved 2 March 2013.
- "Human Genome Browser". Genome Bioinformatics Group of UC Santa Cruz.
- "Homo sapiens asunder spermatogenesis regulator (ASUN), mRNA". NCBI.
- "Homo sapiens transmembrane 7 superfamily member 3 (TM7SF3), mRNA". NCBI.
- "SDSC Biology WorkBench". San Diego Supercomputer Center.
- Petersen, Thomas Nordahl; Søren Brunak; Gunnar von Heijne; Henrik Nielsen (2011). "SignalP 4.0: discriminating signal peptides from transmembrane regions". Nature Methods. 8 (10): 785–786. doi:10.1038/nmeth.1701. PMID 21959131. S2CID 16509924.
- Edgar, R; Domrachev M; Lash AE (Jan 2002). "Gene Expression Omnibus: NCBI gene expression and hybridization array data repository". Nucleic Acids Res. 30 (1): 207–10. doi:10.1093/nar/30.1.207. PMC 99122. PMID 11752295.
- Baba, N (2012). "The aryl hydrocarbon receptor (ahr) ligand vaf347 selectively acts on monocytes and naïve cd4+ th cells to promote the development of il-22-secreting th cells". Human Immunology. 73 (8): 795–800. doi:10.1016/j.humimm.2012.05.002. PMID 22609446.
- "STRING Database".
- Kim; et al. (2012). "Association between fgfr1op2/wit3.0 polymorphisms and residual ridge resorption of mandible in korean population". PLOS ONE. 7 (8): e42734. doi:10.1371/journal.pone.0042734. PMC 3412816. PMID 22880093.
External links
| Wikimedia Commons has media related to FGFR1OP2. |
- Homo sapiens FGFR1 oncogene partner 2 (FGFR1OP2), transcript variant 1, mRNA (NCBI)
- Homo sapiens FGFR1 oncogene partner 2 isoform 1 (NCBI)
- FGFR1OP2 human gene location in the UCSC Genome Browser.
- FGFR1OP2 human gene details in the UCSC Genome Browser.