Sponge
Sponges, the members of the phylum Porifera (/pəˈrɪfərə/; meaning "pore bearer"), are a basal Metazoa (animal) clade as a sister of the Diploblasts.[3][4][5][6][7] They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells. The branch of zoology that studies sponges is known as spongiology.[8]
| Porifera | |
|---|---|
.jpg.webp) | |
| A stove-pipe sponge | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Porifera Grant, 1836 |
| Type species | |
| Amphimedon queenslandica[1] | |
| Classes | |
| Synonyms | |
Sponges have unspecialized cells that can transform into other types and that often migrate between the main cell layers and the mesohyl in the process. Sponges do not have nervous, digestive or circulatory systems. Instead, most rely on maintaining a constant water flow through their bodies to obtain food and oxygen and to remove wastes. Sponges were first to branch off the evolutionary tree from the common ancestor of all animals, making them the sister group of all other animals.[3]
Etymology
The term sponge derives from the Ancient Greek word σπόγγος (spóngos).[9]
Overview

Sponges are similar to other animals in that they are multicellular, heterotrophic, lack cell walls and produce sperm cells. Unlike other animals, they lack true tissues[10] and organs.[11] Some of them are radially symmetrical, but most are asymmetrical. The shapes of their bodies are adapted for maximal efficiency of water flow through the central cavity, where the water deposits nutrients and then leaves through a hole called the osculum. Many sponges have internal skeletons of spongin and/or spicules (skeletal-like fragments) of calcium carbonate or silicon dioxide.[10] All sponges are sessile aquatic animals, meaning that they attach to an underwater surface and remain fixed in place (i.e., do not travel). Although there are freshwater species, the great majority are marine (salt-water) species, ranging in habitat from tidal zones to depths exceeding 8,800 m (5.5 mi).
Although most of the approximately 5,000–10,000 known species of sponges feed on bacteria and other microscopic food in the water, some host photosynthesizing microorganisms as endosymbionts, and these alliances often produce more food and oxygen than they consume. A few species of sponges that live in food-poor environments have evolved as carnivores that prey mainly on small crustaceans.[12]
Most species use sexual reproduction, releasing sperm cells into the water to fertilize ova that in some species are released and in others are retained by the "mother." The fertilized eggs develop into larvae, which swim off in search of places to settle.[13] Sponges are known for regenerating from fragments that are broken off, although this only works if the fragments include the right types of cells. A few species reproduce by budding. When environmental conditions become less hospitable to the sponges, for example as temperatures drop, many freshwater species and a few marine ones produce gemmules, "survival pods" of unspecialized cells that remain dormant until conditions improve; they then either form completely new sponges or recolonize the skeletons of their parents.[14]
In most sponges, an internal gelatinous matrix called mesohyl functions as an endoskeleton, and it is the only skeleton in soft sponges that encrust such hard surfaces as rocks. More commonly, the mesohyl is stiffened by mineral spicules, by spongin fibers, or both. Demosponges use spongin; many species have silica spicules, whereas some species have calcium carbonate exoskeletons. Demosponges constitute about 90% of all known sponge species, including all freshwater ones, and they have the widest range of habitats. Calcareous sponges, which have calcium carbonate spicules and, in some species, calcium carbonate exoskeletons, are restricted to relatively shallow marine waters where production of calcium carbonate is easiest.[15] The fragile glass sponges, with "scaffolding" of silica spicules, are restricted to polar regions and the ocean depths where predators are rare. Fossils of all of these types have been found in rocks dated from 580 million years ago. In addition Archaeocyathids, whose fossils are common in rocks from 530 to 490 million years ago, are now regarded as a type of sponge.
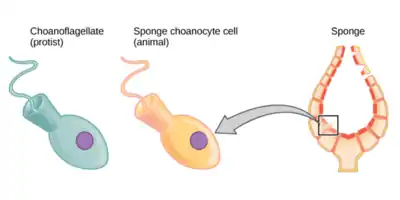
The single-celled choanoflagellates resemble the choanocyte cells of sponges which are used to drive their water flow systems and capture most of their food. This along with phylogenetic studies of ribosomal molecules have been used as morphological evidence to suggest sponges are the sister group to the rest of animals.[17] Some studies have shown that sponges do not form a monophyletic group, in other words do not include all and only the descendants of a common ancestor. Recent phylogenetic analyses suggested that comb jellies rather than sponges are the sister group to the rest of animals.[18][19][20][21] However reanalysis of the data showed that the computer algorithms used for analysis were misled by the presence of specific ctenophore genes that were markedly different from those of other species, leaving sponges as either the sister group to all other animals, or an ancestral paraphyletic grade.[22][23]
The few species of demosponge that have entirely soft fibrous skeletons with no hard elements have been used by humans over thousands of years for several purposes, including as padding and as cleaning tools. By the 1950s, though, these had been overfished so heavily that the industry almost collapsed, and most sponge-like materials are now synthetic. Sponges and their microscopic endosymbionts are now being researched as possible sources of medicines for treating a wide range of diseases. Dolphins have been observed using sponges as tools while foraging.[24]
Distinguishing features
Sponges constitute the phylum Porifera, and have been defined as sessile metazoans (multicelled immobile animals) that have water intake and outlet openings connected by chambers lined with choanocytes, cells with whip-like flagella.[25] However, a few carnivorous sponges have lost these water flow systems and the choanocytes.[26][27] All known living sponges can remold their bodies, as most types of their cells can move within their bodies and a few can change from one type to another.[27][28]
Even if a few sponges are able to produce mucus – which acts as a microbial barrier in all other animals – no sponge with the ability to secrete a functional mucus layer has been recorded. Without such a mucus layer their living tissue is covered by a layer of microbial symbionts, which can contribute up to 40–50% of the sponge wet mass. This inability to prevent microbes from penetrating their porous tissue could be a major reason why they have never evolved a more complex anatomy.[29]
Like cnidarians (jellyfish, etc.) and ctenophores (comb jellies), and unlike all other known metazoans, sponges' bodies consist of a non-living jelly-like mass (mesohyl) sandwiched between two main layers of cells.[30][31] Cnidarians and ctenophores have simple nervous systems, and their cell layers are bound by internal connections and by being mounted on a basement membrane (thin fibrous mat, also known as "basal lamina").[31] Sponges have no nervous systems, their middle jelly-like layers have large and varied populations of cells, and some types of cells in their outer layers may move into the middle layer and change their functions.[28]
| Sponges[28][30] | Cnidarians and ctenophores[31] | |
|---|---|---|
| Nervous system | No | Yes, simple |
| Cells in each layer bound together | No, except that Homoscleromorpha have basement membranes.[32] | Yes: inter-cell connections; basement membranes |
| Number of cells in middle "jelly" layer | Many | Few |
| Cells in outer layers can move inwards and change functions | Yes | No |
Basic structure
Cell types
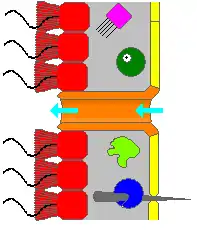
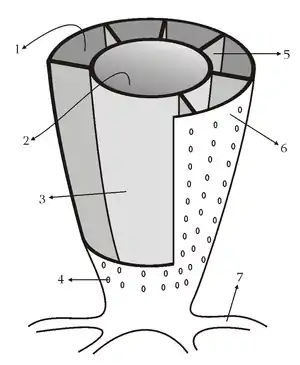
A sponge's body is hollow and is held in shape by the mesohyl, a jelly-like substance made mainly of collagen and reinforced by a dense network of fibers also made of collagen. The inner surface is covered with choanocytes, cells with cylindrical or conical collars surrounding one flagellum per choanocyte. The wave-like motion of the whip-like flagella drives water through the sponge's body. All sponges have ostia, channels leading to the interior through the mesohyl, and in most sponges these are controlled by tube-like porocytes that form closable inlet valves. Pinacocytes, plate-like cells, form a single-layered external skin over all other parts of the mesohyl that are not covered by choanocytes, and the pinacocytes also digest food particles that are too large to enter the ostia,[28][30] while those at the base of the animal are responsible for anchoring it.[30]
Other types of cell live and move within the mesohyl:[28][30]
- Lophocytes are amoeba-like cells that move slowly through the mesohyl and secrete collagen fibres.
- Collencytes are another type of collagen-producing cell.
- Rhabdiferous cells secrete polysaccharides that also form part of the mesohyl.
- Oocytes and spermatocytes are reproductive cells.
- Sclerocytes secrete the mineralized spicules ("little spines") that form the skeletons of many sponges and in some species provide some defense against predators.
- In addition to or instead of sclerocytes, demosponges have spongocytes that secrete a form of collagen that polymerizes into spongin, a thick fibrous material that stiffens the mesohyl.
- Myocytes ("muscle cells") conduct signals and cause parts of the animal to contract.
- "Grey cells" act as sponges' equivalent of an immune system.
- Archaeocytes (or amoebocytes) are amoeba-like cells that are totipotent, in other words each is capable of transformation into any other type of cell. They also have important roles in feeding and in clearing debris that block the ostia.
Many larval sponges possess neuron-less eyes that are based on cryptochromes. They mediate phototaxic behavior.[35]
Glass sponges' syncytia
Glass sponges present a distinctive variation on this basic plan. Their spicules, which are made of silica, form a scaffolding-like framework between whose rods the living tissue is suspended like a cobweb that contains most of the cell types.[28] This tissue is a syncytium that in some ways behaves like many cells that share a single external membrane, and in others like a single cell with multiple nuclei. The mesohyl is absent or minimal. The syncytium's cytoplasm, the soupy fluid that fills the interiors of cells, is organized into "rivers" that transport nuclei, organelles ("organs" within cells) and other substances.[38] Instead of choanocytes, they have further syncytia, known as choanosyncytia, which form bell-shaped chambers where water enters via perforations. The insides of these chambers are lined with "collar bodies", each consisting of a collar and flagellum but without a nucleus of its own. The motion of the flagella sucks water through passages in the "cobweb" and expels it via the open ends of the bell-shaped chambers.[28]
Some types of cells have a single nucleus and membrane each, but are connected to other single-nucleus cells and to the main syncytium by "bridges" made of cytoplasm. The sclerocytes that build spicules have multiple nuclei, and in glass sponge larvae they are connected to other tissues by cytoplasm bridges; such connections between sclerocytes have not so far been found in adults, but this may simply reflect the difficulty of investigating such small-scale features. The bridges are controlled by "plugged junctions" that apparently permit some substances to pass while blocking others.[38]
Water flow and body structures
Most sponges work rather like chimneys: they take in water at the bottom and eject it from the osculum ("little mouth") at the top. Since ambient currents are faster at the top, the suction effect that they produce by Bernoulli's principle does some of the work for free. Sponges can control the water flow by various combinations of wholly or partially closing the osculum and ostia (the intake pores) and varying the beat of the flagella, and may shut it down if there is a lot of sand or silt in the water.[28]
Although the layers of pinacocytes and choanocytes resemble the epithelia of more complex animals, they are not bound tightly by cell-to-cell connections or a basal lamina (thin fibrous sheet underneath). The flexibility of these layers and re-modeling of the mesohyl by lophocytes allow the animals to adjust their shapes throughout their lives to take maximum advantage of local water currents.[41]
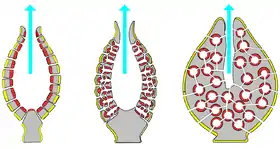
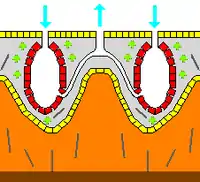
The simplest body structure in sponges is a tube or vase shape known as "asconoid", but this severely limits the size of the animal. The body structure is characterized by a stalk-like spongocoel surrounded by a single layer of choanocytes. If it is simply scaled up, the ratio of its volume to surface area increases, because surface increases as the square of length or width while volume increases proportionally to the cube. The amount of tissue that needs food and oxygen is determined by the volume, but the pumping capacity that supplies food and oxygen depends on the area covered by choanocytes. Asconoid sponges seldom exceed 1 mm (0.039 in) in diameter.[28]
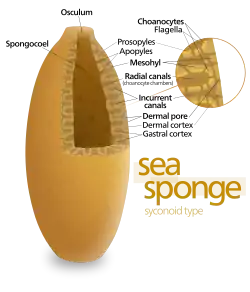
Some sponges overcome this limitation by adopting the "syconoid" structure, in which the body wall is pleated. The inner pockets of the pleats are lined with choanocytes, which connect to the outer pockets of the pleats by ostia. This increase in the number of choanocytes and hence in pumping capacity enables syconoid sponges to grow up to a few centimeters in diameter.
The "leuconoid" pattern boosts pumping capacity further by filling the interior almost completely with mesohyl that contains a network of chambers lined with choanocytes and connected to each other and to the water intakes and outlet by tubes. Leuconid sponges grow to over 1 m (3.3 ft) in diameter, and the fact that growth in any direction increases the number of choanocyte chambers enables them to take a wider range of forms, for example "encrusting" sponges whose shapes follow those of the surfaces to which they attach. All freshwater and most shallow-water marine sponges have leuconid bodies. The networks of water passages in glass sponges are similar to the leuconid structure.[28] In all three types of structure the cross-section area of the choanocyte-lined regions is much greater than that of the intake and outlet channels. This makes the flow slower near the choanocytes and thus makes it easier for them to trap food particles.[28] For example, in Leuconia, a small leuconoid sponge about 10 centimetres (3.9 in) tall and 1 centimetre (0.39 in) in diameter, water enters each of more than 80,000 intake canals at 6 cm per minute. However, because Leuconia has more than 2 million flagellated chambers whose combined diameter is much greater than that of the canals, water flow through chambers slows to 3.6 cm per hour, making it easy for choanocytes to capture food. All the water is expelled through a single osculum at about 8.5 cm per second, fast enough to carry waste products some distance away.[42]
Skeleton
In zoology a skeleton is any fairly rigid structure of an animal, irrespective of whether it has joints and irrespective of whether it is biomineralized. The mesohyl functions as an endoskeleton in most sponges, and is the only skeleton in soft sponges that encrust hard surfaces such as rocks. More commonly the mesohyl is stiffened by mineral spicules, by spongin fibers or both. Spicules, which are present in most but not all species,[43] may be made of silica or calcium carbonate, and vary in shape from simple rods to three-dimensional "stars" with up to six rays. Spicules are produced by sclerocyte cells,[28] and may be separate, connected by joints, or fused.[27]
Some sponges also secrete exoskeletons that lie completely outside their organic components. For example, sclerosponges ("hard sponges") have massive calcium carbonate exoskeletons over which the organic matter forms a thin layer with choanocyte chambers in pits in the mineral. These exoskeletons are secreted by the pinacocytes that form the animals' skins.[28]
Vital functions

Movement
Although adult sponges are fundamentally sessile animals, some marine and freshwater species can move across the sea bed at speeds of 1–4 mm (0.039–0.157 in) per day, as a result of amoeba-like movements of pinacocytes and other cells. A few species can contract their whole bodies, and many can close their oscula and ostia. Juveniles drift or swim freely, while adults are stationary.[28]
Respiration, feeding and excretion

Sponges do not have distinct circulatory, respiratory, digestive, and excretory systems – instead the water flow system supports all these functions. They filter food particles out of the water flowing through them. Particles larger than 50 micrometers cannot enter the ostia and pinacocytes consume them by phagocytosis (engulfing and internal digestion). Particles from 0.5 μm to 50 μm are trapped in the ostia, which taper from the outer to inner ends. These particles are consumed by pinacocytes or by archaeocytes which partially extrude themselves through the walls of the ostia. Bacteria-sized particles, below 0.5 micrometers, pass through the ostia and are caught and consumed by choanocytes.[28] Since the smallest particles are by far the most common, choanocytes typically capture 80% of a sponge's food supply.[44] Archaeocytes transport food packaged in vesicles from cells that directly digest food to those that do not. At least one species of sponge has internal fibers that function as tracks for use by nutrient-carrying archaeocytes,[28] and these tracks also move inert objects.[30]
It used to be claimed that glass sponges could live on nutrients dissolved in sea water and were very averse to silt.[45] However, a study in 2007 found no evidence of this and concluded that they extract bacteria and other micro-organisms from water very efficiently (about 79%) and process suspended sediment grains to extract such prey.[46] Collar bodies digest food and distribute it wrapped in vesicles that are transported by dynein "motor" molecules along bundles of microtubules that run throughout the syncytium.[28]
Sponges' cells absorb oxygen by diffusion from water into cells as water flows through body, into which carbon dioxide and other soluble waste products such as ammonia also diffuse. Archeocytes remove mineral particles that threaten to block the ostia, transport them through the mesohyl and generally dump them into the outgoing water current, although some species incorporate them into their skeletons.[28]
Carnivorous sponges

A few species that live in waters where the supply of food particles is very poor prey on crustaceans and other small animals. So far only 137 species have been discovered.[48] Most belong to the family Cladorhizidae, but a few members of the Guitarridae and Esperiopsidae are also carnivores.[49] In most cases little is known about how they actually capture prey, although some species are thought to use either sticky threads or hooked spicules.[49][50] Most carnivorous sponges live in deep waters, up to 8,840 m (5.49 mi),[51] and the development of deep-ocean exploration techniques is expected to lead to the discovery of several more.[28][49] However, one species has been found in Mediterranean caves at depths of 17–23 m (56–75 ft), alongside the more usual filter feeding sponges. The cave-dwelling predators capture crustaceans under 1 mm (0.039 in) long by entangling them with fine threads, digest them by enveloping them with further threads over the course of a few days, and then return to their normal shape; there is no evidence that they use venom.[51]
Most known carnivorous sponges have completely lost the water flow system and choanocytes. However, the genus Chondrocladia uses a highly modified water flow system to inflate balloon-like structures that are used for capturing prey.[49][52]
Endosymbionts
Freshwater sponges often host green algae as endosymbionts within archaeocytes and other cells, and benefit from nutrients produced by the algae. Many marine species host other photosynthesizing organisms, most commonly cyanobacteria but in some cases dinoflagellates. Symbiotic cyanobacteria may form a third of the total mass of living tissue in some sponges, and some sponges gain 48% to 80% of their energy supply from these micro-organisms.[28] In 2008 a University of Stuttgart team reported that spicules made of silica conduct light into the mesohyl, where the photosynthesizing endosymbionts live.[53] Sponges that host photosynthesizing organisms are most common in waters with relatively poor supplies of food particles, and often have leafy shapes that maximize the amount of sunlight they collect.[30]
A recently discovered carnivorous sponge that lives near hydrothermal vents hosts methane-eating bacteria, and digests some of them.[30]
"Immune" system
Sponges do not have the complex immune systems of most other animals. However, they reject grafts from other species but accept them from other members of their own species. In a few marine species, gray cells play the leading role in rejection of foreign material. When invaded, they produce a chemical that stops movement of other cells in the affected area, thus preventing the intruder from using the sponge's internal transport systems. If the intrusion persists, the grey cells concentrate in the area and release toxins that kill all cells in the area. The "immune" system can stay in this activated state for up to three weeks.[30]
Asexual

Sponges have three asexual methods of reproduction: after fragmentation; by budding; and by producing gemmules. Fragments of sponges may be detached by currents or waves. They use the mobility of their pinacocytes and choanocytes and reshaping of the mesohyl to re-attach themselves to a suitable surface and then rebuild themselves as small but functional sponges over the course of several days. The same capabilities enable sponges that have been squeezed through a fine cloth to regenerate.[54] A sponge fragment can only regenerate if it contains both collencytes to produce mesohyl and archeocytes to produce all the other cell types.[44] A very few species reproduce by budding.[55]
Gemmules are "survival pods" which a few marine sponges and many freshwater species produce by the thousands when dying and which some, mainly freshwater species, regularly produce in autumn. Spongocytes make gemmules by wrapping shells of spongin, often reinforced with spicules, round clusters of archeocytes that are full of nutrients.[56] Freshwater gemmules may also include phytosynthesizing symbionts.[57] The gemmules then become dormant, and in this state can survive cold, drying out, lack of oxygen and extreme variations in salinity.[28] Freshwater gemmules often do not revive until the temperature drops, stays cold for a few months and then reaches a near-"normal" level.[57] When a gemmule germinates, the archeocytes round the outside of the cluster transform into pinacocytes, a membrane over a pore in the shell bursts, the cluster of cells slowly emerges, and most of the remaining archeocytes transform into other cell types needed to make a functioning sponge. Gemmules from the same species but different individuals can join forces to form one sponge.[58] Some gemmules are retained within the parent sponge, and in spring it can be difficult to tell whether an old sponge has revived or been "recolonized" by its own gemmules.[57]
Sexual
Most sponges are hermaphrodites (function as both sexes simultaneously), although sponges have no gonads (reproductive organs). Sperm are produced by choanocytes or entire choanocyte chambers that sink into the mesohyl and form spermatic cysts while eggs are formed by transformation of archeocytes, or of choanocytes in some species. Each egg generally acquires a yolk by consuming "nurse cells". During spawning, sperm burst out of their cysts and are expelled via the osculum. If they contact another sponge of the same species, the water flow carries them to choanocytes that engulf them but, instead of digesting them, metamorphose to an ameboid form and carry the sperm through the mesohyl to eggs, which in most cases engulf the carrier and its cargo.[59]
A few species release fertilized eggs into the water, but most retain the eggs until they hatch. There are four types of larvae, but all are balls of cells with an outer layer of cells whose flagellae or cilia enable the larvae to move. After swimming for a few days the larvae sink and crawl until they find a place to settle. Most of the cells transform into archeocytes and then into the types appropriate for their locations in a miniature adult sponge.[59]
Glass sponge embryos start by dividing into separate cells, but once 32 cells have formed they rapidly transform into larvae that externally are ovoid with a band of cilia round the middle that they use for movement, but internally have the typical glass sponge structure of spicules with a cobweb-like main syncitium draped around and between them and choanosyncytia with multiple collar bodies in the center. The larvae then leave their parents' bodies.[60]
Life cycle
Sponges in temperate regions live for at most a few years, but some tropical species and perhaps some deep-ocean ones may live for 200 years or more. Some calcified demosponges grow by only 0.2 mm (0.0079 in) per year and, if that rate is constant, specimens 1 m (3.3 ft) wide must be about 5,000 years old. Some sponges start sexual reproduction when only a few weeks old, while others wait until they are several years old.[28]
Coordination of activities
Adult sponges lack neurons or any other kind of nervous tissue. However, most species have the ability to perform movements that are coordinated all over their bodies, mainly contractions of the pinacocytes, squeezing the water channels and thus expelling excess sediment and other substances that may cause blockages. Some species can contract the osculum independently of the rest of the body. Sponges may also contract in order to reduce the area that is vulnerable to attack by predators. In cases where two sponges are fused, for example if there is a large but still unseparated bud, these contraction waves slowly become coordinated in both of the "Siamese twins". The coordinating mechanism is unknown, but may involve chemicals similar to neurotransmitters.[61] However, glass sponges rapidly transmit electrical impulses through all parts of the syncytium, and use this to halt the motion of their flagella if the incoming water contains toxins or excessive sediment.[28] Myocytes are thought to be responsible for closing the osculum and for transmitting signals between different parts of the body.[30]
Sponges contain genes very similar to those that contain the "recipe" for the post-synaptic density, an important signal-receiving structure in the neurons of all other animals. However, in sponges these genes are only activated in "flask cells" that appear only in larvae and may provide some sensory capability while the larvae are swimming. This raises questions about whether flask cells represent the predecessors of true neurons or are evidence that sponges' ancestors had true neurons but lost them as they adapted to a sessile lifestyle.[62]
Ecology
.jpg.webp)
Habitats
Sponges are worldwide in their distribution, living in a wide range of ocean habitats, from the polar regions to the tropics.[44] Most live in quiet, clear waters, because sediment stirred up by waves or currents would block their pores, making it difficult for them to feed and breathe.[45] The greatest numbers of sponges are usually found on firm surfaces such as rocks, but some sponges can attach themselves to soft sediment by means of a root-like base.[63]
Sponges are more abundant but less diverse in temperate waters than in tropical waters, possibly because organisms that prey on sponges are more abundant in tropical waters.[64] Glass sponges are the most common in polar waters and in the depths of temperate and tropical seas, as their very porous construction enables them to extract food from these resource-poor waters with the minimum of effort. Demosponges and calcareous sponges are abundant and diverse in shallower non-polar waters.[65]
The different classes of sponge live in different ranges of habitat:
| Water type[30] | Depth[30] | Type of surface[30] | |
|---|---|---|---|
| Calcarea | Marine | less than 100 m (330 ft) | Hard |
| Glass sponges | Marine | Deep | Soft or firm sediment |
| Demosponges | Marine, brackish; and about 150 freshwater species[28] | Inter-tidal to abyssal;[30] a carnivorous demosponge has been found at 8,840 m (5.49 mi)[51] | Any |
As primary producers
Sponges with photosynthesizing endosymbionts produce up to three times more oxygen than they consume, as well as more organic matter than they consume. Such contributions to their habitats' resources are significant along Australia's Great Barrier Reef but relatively minor in the Caribbean.[44]
Defenses


Many sponges shed spicules, forming a dense carpet several meters deep that keeps away echinoderms which would otherwise prey on the sponges.[44] They also produce toxins that prevent other sessile organisms such as bryozoans or sea squirts from growing on or near them, making sponges very effective competitors for living space. One of many examples includes ageliferin.
A few species, the Caribbean fire sponge Tedania ignis, cause a severe rash in humans who handle them.[28] Turtles and some fish feed mainly on sponges. It is often said that sponges produce chemical defenses against such predators.[28] However, experiments have been unable to establish a relationship between the toxicity of chemicals produced by sponges and how they taste to fish, which would diminish the usefulness of chemical defenses as deterrents. Predation by fish may even help to spread sponges by detaching fragments.[30] However, some studies have shown fish showing a preference for non chemically defended sponges,[66] and another study found that high levels of coral predation did predict the presence of chemically defended species.[67]
Glass sponges produce no toxic chemicals, and live in very deep water where predators are rare.[45]
Predation
Sponge flies, also known as spongilla-flies (Neuroptera, Sisyridae), are specialist predators of freshwater sponges. The female lays her eggs on vegetation overhanging water. The larvae hatch and drop into the water where they seek out sponges to feed on. They use their elongated mouthparts to pierce the sponge and suck the fluids within. The larvae of some species cling to the surface of the sponge while others take refuge in the sponge's internal cavities. The fully grown larvae leave the water and spin a cocoon in which to pupate.[68]
Bioerosion
The Caribbean chicken-liver sponge Chondrilla nucula secretes toxins that kill coral polyps, allowing the sponges to grow over the coral skeletons.[28] Others, especially in the family Clionaidae, use corrosive substances secreted by their archeocytes to tunnel into rocks, corals and the shells of dead mollusks.[28] Sponges may remove up to 1 m (3.3 ft) per year from reefs, creating visible notches just below low-tide level.[44]
Diseases
Caribbean sponges of the genus Aplysina suffer from Aplysina red band syndrome. This causes Aplysina to develop one or more rust-colored bands, sometimes with adjacent bands of necrotic tissue. These lesions may completely encircle branches of the sponge. The disease appears to be contagious and impacts approximately 10 percent of A. cauliformis on Bahamian reefs.[69] The rust-colored bands are caused by a cyanobacterium, but it is unknown whether this organism actually causes the disease.[69][70]
Collaboration with other organisms
In addition to hosting photosynthesizing endosymbionts,[28] sponges are noted for their wide range of collaborations with other organisms. The relatively large encrusting sponge Lissodendoryx colombiensis is most common on rocky surfaces, but has extended its range into seagrass meadows by letting itself be surrounded or overgrown by seagrass sponges, which are distasteful to the local starfish and therefore protect Lissodendoryx against them; in return the seagrass sponges get higher positions away from the sea-floor sediment.[71]
Shrimps of the genus Synalpheus form colonies in sponges, and each shrimp species inhabits a different sponge species, making Synalpheus one of the most diverse crustacean genera. Specifically, Synalpheus regalis utilizes the sponge not only as a food source, but also as a defense against other shrimp and predators.[72] As many as 16,000 individuals inhabit a single loggerhead sponge, feeding off the larger particles that collect on the sponge as it filters the ocean to feed itself.[73]
Sponge loop
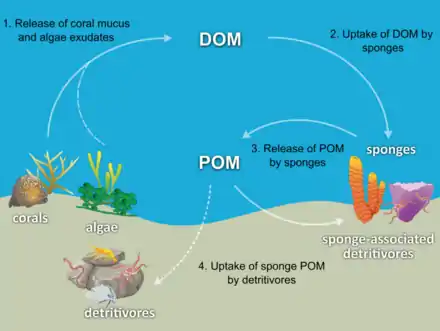
Most sponges are detritivores which filter organic debris particles and microscopic life forms from ocean water. In particular, sponges occupy an important role as detritivores in coral reef food webs by recycling detritus to higher trophic levels.[74]
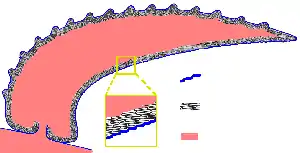
The hypothesis has been made that coral reef sponges facilitate the transfer of coral-derived organic matter to their associated detritivores via the production of sponge detritus, as shown in the diagram. Several sponge species are able to convert coral-derived DOM into sponge detritus,[77][78] and transfer organic matter produced by corals further up the reef food web. Corals release organic matter as both dissolved and particulate mucus,[79][80][81][82] as well as cellular material such as expelled Symbiodinium.[83][84][74]
Organic matter could be transferred from corals to sponges by all these pathways, but DOM likely makes up the largest fraction, as the majority (56 to 80%) of coral mucus dissolves in the water column,[80] and coral loss of fixed carbon due to expulsion of Symbiodinium is typically negligible (0.01%)[83] compared with mucus release (up to ~40%).[85][86] Coral-derived organic matter could also be indirectly transferred to sponges via bacteria, which can also consume coral mucus.[87][88][89][74]
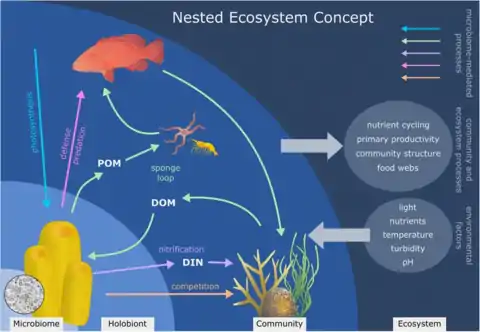
Sponge holobiont
Besides a one to one symbiotic relationship, it is possible for a host to become symbiotic with a microbial consortia. Sponges are able to host a wide range of microbial communities that can also be very specific. The microbial communities that form a symbiotic relationship with the sponge can amount to as much as 35% of the biomass of its host.[91] The term for this specific symbiotic relationship, where a microbial consortia pairs with a host is called a holobiotic relationship. The sponge as well as the microbial community associated with it will produce a large range of secondary metabolites that help protect it against predators through mechanisms such as chemical defense.[92]
Some of these relationships include endosymbionts within bacteriocyte cells, and cyanobacteria or microalgae found below the pinacoderm cell layer where they are able to receive the highest amount of light, used for phototrophy. They can host over 50 different microbial phyla and candidate phyla, including Alphaprotoebacteria, Actinobacteria, Chloroflexi, Nitrospirae, Cyanobacteria, the taxa Gamma-, the candidate phylum Poribacteria, and Thaumarchaea.[92]
Systematics and evolutionary history
Taxonomy
Linnaeus, who classified most kinds of sessile animals as belonging to the order Zoophyta in the class Vermes, mistakenly identified the genus Spongia as plants in the order Algae.[93] For a long time thereafter sponges were assigned to a separate subkingdom, Parazoa ("beside the animals"), separate from the Eumetazoa which formed the rest of the kingdom Animalia.[94] They have been regarded as a paraphyletic phylum, from which the higher animals have evolved.[95] Other research indicates Porifera is monophyletic.[96]
The phylum Porifera is further divided into classes mainly according to the composition of their skeletons:[27][44]
- Hexactinellida (glass sponges) have silicate spicules, the largest of which have six rays and may be individual or fused.[27] The main components of their bodies are syncytia in which large numbers of cell share a single external membrane.[44]
- Calcarea have skeletons made of calcite, a form of calcium carbonate, which may form separate spicules or large masses. All the cells have a single nucleus and membrane.[44]
- Most Demospongiae have silicate spicules or spongin fibers or both within their soft tissues. However, a few also have massive external skeletons made of aragonite, another form of calcium carbonate.[27][44] All the cells have a single nucleus and membrane.[44]
- Archeocyatha are known only as fossils from the Cambrian period.[94]
In the 1970s, sponges with massive calcium carbonate skeletons were assigned to a separate class, Sclerospongiae, otherwise known as "coralline sponges".[97] However, in the 1980s it was found that these were all members of either the Calcarea or the Demospongiae.[98]
So far scientific publications have identified about 9,000 poriferan species,[44] of which: about 400 are glass sponges; about 500 are calcareous species; and the rest are demosponges.[28] However, some types of habitat, vertical rock and cave walls and galleries in rock and coral boulders, have been investigated very little, even in shallow seas.[44]
Classes
Sponges were traditionally distributed in three classes: calcareous sponges (Calcarea), glass sponges (Hexactinellida) and demosponges (Demospongiae). However, studies have shown that the Homoscleromorpha, a group thought to belong to the Demospongiae, is actually phylogenetically well separated.[99] Therefore, they have recently been recognized as the fourth class of sponges.[100][101]
Sponges are divided into classes mainly according to the composition of their skeletons:[30] These are arranged in evolutionary order as shown below in ascending order of their evolution from top to bottom:
| Type of cells[30] | Spicules[30] | Spongin fibers[30] | Massive exoskeleton[44] | Body form[30] | |
|---|---|---|---|---|---|
| Hexactinellida | Mostly syncytia in all species | Silica May be individual or fused | Never | Never | Leuconoid |
| Demospongiae | Single nucleus, single external membrane | Silica | In many species | In some species. Made of aragonite if present.[27][44] | Leuconoid |
| Calcarea | Single nucleus, single external membrane | Calcite May be individual or large masses | Never | Common. Made of calcite if present. | Asconoid, syconoid, leuconoid or solenoid[102] |
| Homoscleromorpha | Single nucleus, single external membrane | Silica | In many species | Never | Sylleibid or leuconoid |
Fossil record

Although molecular clocks and biomarkers suggest sponges existed well before the Cambrian explosion of life, silica spicules like those of demosponges are absent from the fossil record until the Cambrian.[103] One unsubstantiated report exists of spicules in rocks dated around 750 million years ago.[104] Well-preserved fossil sponges from about 580 million years ago in the Ediacaran period have been found in the Doushantuo Formation. These fossils, which include spicules, pinacocytes, porocytes, archeocytes, sclerocytes and the internal cavity, have been classified as demosponges. Fossils of glass sponges have been found from around 540 million years ago in rocks in Australia, China and Mongolia.[105] Early Cambrian sponges from Mexico belonging to the genus Kiwetinokia show evidence of fusion of several smaller spicules to form a single large spicule.[106] Calcium carbonate spicules of calcareous sponges have been found in Early Cambrian rocks from about 530 to 523 million years ago in Australia. Other probable demosponges have been found in the Early Cambrian Chengjiang fauna, from 525 to 520 million years ago.[107] Freshwater sponges appear to be much younger, as the earliest known fossils date from the Mid-Eocene period about 48 to 40 million years ago.[105] Although about 90% of modern sponges are demosponges, fossilized remains of this type are less common than those of other types because their skeletons are composed of relatively soft spongin that does not fossilize well.[108] Earliest sponge symbionts are known from the early Silurian.[109]
A chemical tracer is 24-isopropylcholestane, which is a stable derivative of 24-isopropylcholesterol, which is said to be produced by demosponges but not by eumetazoans ("true animals", i.e. cnidarians and bilaterians). Since choanoflagellates are thought to be animals' closest single-celled relatives, a team of scientists examined the biochemistry and genes of one choanoflagellate species. They concluded that this species could not produce 24-isopropylcholesterol but that investigation of a wider range of choanoflagellates would be necessary in order to prove that the fossil 24-isopropylcholestane could only have been produced by demosponges.[110] Although a previous publication reported traces of the chemical 24-isopropylcholestane in ancient rocks dating to 1,800 million years ago,[111] recent research using a much more accurately dated rock series has revealed that these biomarkers only appear before the end of the Marinoan glaciation approximately 635 million years ago,[112] and that "Biomarker analysis has yet to reveal any convincing evidence for ancient sponges pre-dating the first globally extensive Neoproterozoic glacial episode (the Sturtian, ~713 million years ago in Oman)". While it has been argued that this 'sponge biomarker' could have originated from marine algae, recent research suggests that the algae's ability to produce this biomarker evolved only in the Carboniferous; as such, the biomarker remains strongly supportive of the presence of demosponges in the Cryogenian.[113][114][115]
Archaeocyathids, which some classify as a type of coralline sponge, are very common fossils in rocks from the Early Cambrian about 530 to 520 million years ago, but apparently died out by the end of the Cambrian 490 million years ago.[107] It has been suggested that they were produced by: sponges; cnidarians; algae; foraminiferans; a completely separate phylum of animals, Archaeocyatha; or even a completely separate kingdom of life, labeled Archaeata or Inferibionta. Since the 1990s archaeocyathids have been regarded as a distinctive group of sponges.[94]
It is difficult to fit chancelloriids into classifications of sponges or more complex animals. An analysis in 1996 concluded that they were closely related to sponges on the grounds that the detailed structure of chancellorid sclerites ("armor plates") is similar to that of fibers of spongin, a collagen protein, in modern keratose (horny) demosponges such as Darwinella.[117] However, another analysis in 2002 concluded that chancelloriids are not sponges and may be intermediate between sponges and more complex animals, among other reasons because their skins were thicker and more tightly connected than those of sponges.[118] In 2008 a detailed analysis of chancelloriids' sclerites concluded that they were very similar to those of halkieriids, mobile bilaterian animals that looked like slugs in chain mail and whose fossils are found in rocks from the very Early Cambrian to the Mid Cambrian. If this is correct, it would create a dilemma, as it is extremely unlikely that totally unrelated organisms could have developed such similar sclerites independently, but the huge difference in the structures of their bodies makes it hard to see how they could be closely related.[116]
Relationships to other animal groups

Simplified family tree showing calcareous sponges as closest to more complex animals[119] | ||||||||||||||||||||||||||||||||||||
as closest to more complex animals[120] |
In the 1990s sponges were widely regarded as a monophyletic group, all of them having descended from a common ancestor that was itself a sponge, and as the "sister-group" to all other metazoans (multi-celled animals), which themselves form a monophyletic group. On the other hand, some 1990s analyses also revived the idea that animals' nearest evolutionary relatives are choanoflagellates, single-celled organisms very similar to sponges' choanocytes – which would imply that most Metazoa evolved from very sponge-like ancestors and therefore that sponges may not be monophyletic, as the same sponge-like ancestors may have given rise both to modern sponges and to non-sponge members of Metazoa.[119]
Analyses since 2001 have concluded that Eumetazoa (more complex than sponges) are more closely related to particular groups of sponges than to the rest of the sponges. Such conclusions imply that sponges are not monophyletic, because the last common ancestor of all sponges would also be a direct ancestor of the Eumetazoa, which are not sponges. A study in 2001 based on comparisons of ribosome DNA concluded that the most fundamental division within sponges was between glass sponges and the rest, and that Eumetazoa are more closely related to calcareous sponges, those with calcium carbonate spicules, than to other types of sponge.[119] In 2007 one analysis based on comparisons of RNA and another based mainly on comparison of spicules concluded that demosponges and glass sponges are more closely related to each other than either is to calcareous sponges, which in turn are more closely related to Eumetazoa.[105][121]
Other anatomical and biochemical evidence links the Eumetazoa with Homoscleromorpha, a sub-group of demosponges. A comparison in 2007 of nuclear DNA, excluding glass sponges and comb jellies, concluded that: Homoscleromorpha are most closely related to Eumetazoa; calcareous sponges are the next closest; the other demosponges are evolutionary "aunts" of these groups; and the chancelloriids, bag-like animals whose fossils are found in Cambrian rocks, may be sponges.[120] The sperm of Homoscleromorpha share with those of Eumetazoa features that those of other sponges lack. In both Homoscleromorpha and Eumetazoa layers of cells are bound together by attachment to a carpet-like basal membrane composed mainly of "type IV" collagen, a form of collagen not found in other sponges – although the spongin fibers that reinforce the mesohyl of all demosponges is similar to "type IV" collagen.[32]
The analyses described above concluded that sponges are closest to the ancestors of all Metazoa, of all multi-celled animals including both sponges and more complex groups. However, another comparison in 2008 of 150 genes in each of 21 genera, ranging from fungi to humans but including only two species of sponge, suggested that comb jellies (ctenophora) are the most basal lineage of the Metazoa included in the sample. If this is correct, either modern comb jellies developed their complex structures independently of other Metazoa, or sponges' ancestors were more complex and all known sponges are drastically simplified forms. The study recommended further analyses using a wider range of sponges and other simple Metazoa such as Placozoa.[18] The results of such an analysis, published in 2009, suggest that a return to the previous view may be warranted. 'Family trees' constructed using a combination of all available data – morphological, developmental and molecular – concluded that the sponges are in fact a monophyletic group, and with the cnidarians form the sister group to the bilaterians.[122][123]
A very large and internally consistent alignment of 1,719 proteins at the metazoan scale, published in 2017, showed that (i) sponges – represented by Homoscleromorpha, Calcarea, Hexactinellida, and Demospongiae – are monophyletic, (ii) sponges are sister-group to all other multicellular animals, (iii) ctenophores emerge as the second-earliest branching animal lineage, and (iv) placozoans emerge as the third animal lineage, followed by cnidarians sister-group to bilaterians.[5]
Notable spongiologists
- Céline Allewaert
- Patricia Bergquist
- James Scott Bowerbank
- Maurice Burton
- Henry John Carter
- Max Walker de Laubenfels
- Arthur Dendy
- Édouard Placide Duchassaing de Fontbressin
- Randolph Kirkpatrick
- Robert J. Lendlmayer von Lendenfeld
- Edward Alfred Minchin
- Giovanni Domenico Nardo
- Eduard Oscar Schmidt
- Émile Topsent
Use

By dolphins
A report in 1997 described use of sponges as a tool by bottlenose dolphins in Shark Bay in Western Australia. A dolphin will attach a marine sponge to its rostrum, which is presumably then used to protect it when searching for food in the sandy sea bottom.[124] The behavior, known as sponging, has only been observed in this bay, and is almost exclusively shown by females. A study in 2005 concluded that mothers teach the behavior to their daughters, and that all the sponge-users are closely related, suggesting that it is a fairly recent innovation.[24]
By humans

Skeleton
The calcium carbonate or silica spicules of most sponge genera make them too rough for most uses, but two genera, Hippospongia and Spongia, have soft, entirely fibrous skeletons.[125] Early Europeans used soft sponges for many purposes, including padding for helmets, portable drinking utensils and municipal water filters. Until the invention of synthetic sponges, they were used as cleaning tools, applicators for paints and ceramic glazes and discreet contraceptives. However, by the mid-20th century, over-fishing brought both the animals and the industry close to extinction.[126] See also sponge diving.
Many objects with sponge-like textures are now made of substances not derived from poriferans. Synthetic sponges include personal and household cleaning tools, breast implants,[127] and contraceptive sponges.[128] Typical materials used are cellulose foam, polyurethane foam, and less frequently, silicone foam.
The luffa "sponge", also spelled loofah, which is commonly sold for use in the kitchen or the shower, is not derived from an animal but mainly from the fibrous "skeleton" of the sponge gourd (Luffa aegyptiaca, Cucurbitaceae).[129]
Antibiotic compounds
Sponges have medicinal potential due to the presence in sponges themselves or their microbial symbionts of chemicals that may be used to control viruses, bacteria, tumors and fungi.[130][131]
Other biologically active compounds
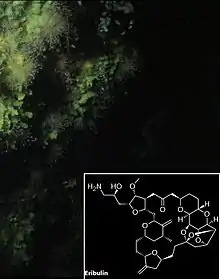
Lacking any protective shell or means of escape, sponges have evolved to synthesize a variety of unusual compounds. One such class is the oxidized fatty acid derivatives called oxylipins. Members of this family have been found to have anti-cancer, anti-bacterial and anti-fungal properties. One example isolated from the Okinawan plakortis sponges, plakoridine A, has shown potential as a cytotoxin to murine lymphoma cells.[132][133]
See also
References
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, et al. (August 2010). "The Amphimedon queenslandica genome and the evolution of animal complexity". Nature. 466 (7307): 720–6. Bibcode:2010Natur.466..720S. doi:10.1038/nature09201. PMC 3130542. PMID 20686567.
- Pajdzińska A (2018). "Animals die more shallowly: they aren't deceased, they're dead. Animals in the polish linguistic worldview and in contemporary life sciences" (PDF). Ethnolinguistic. 29: 147–161. doi:10.17951/et.2017.29.135. Archived from the original (PDF) on 2019-02-26.
- Feuda R, Dohrmann M, Pett W, Philippe H, Rota-Stabelli O, Lartillot N, et al. (December 2017). "Improved Modeling of Compositional Heterogeneity Supports Sponges as Sister to All Other Animals". Current Biology. 27 (24): 3864–3870.e4. doi:10.1016/j.cub.2017.11.008. PMID 29199080.
- Pisani D, Pett W, Dohrmann M, Feuda R, Rota-Stabelli O, Philippe H, et al. (December 2015). "Genomic data do not support comb jellies as the sister group to all other animals". Proceedings of the National Academy of Sciences of the United States of America. 112 (50): 15402–7. Bibcode:2015PNAS..11215402P. doi:10.1073/pnas.1518127112. PMC 4687580. PMID 26621703.
- Simion P, Philippe H, Baurain D, Jager M, Richter DJ, Di Franco A, et al. (April 2017). "A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals" (PDF). Current Biology. 27 (7): 958–967. doi:10.1016/j.cub.2017.02.031. PMID 28318975.
- Giribet G (1 October 2016). "Genomics and the animal tree of life: conflicts and future prospects". Zoologica Scripta. 45: 14–21. doi:10.1111/zsc.12215. ISSN 1463-6409.
- Laumer CE, Gruber-Vodicka H, Hadfield MG, Pearse VB, Riesgo A, Marioni JC, Giribet G (2017-10-11). "Placozoans are eumetazoans related to Cnidaria". bioRxiv 10.1101/200972.
- "Spongiology". Merriam-Webster Dictionary. Retrieved 27 December 2017.
- "Henry George Liddell, Robert Scott, A Greek-English Lexicon".
- Hooper, John (2018). "Structure of Sponges". Queensland Museum. Archived from the original on 26 September 2019. Retrieved 27 September 2019.
- Thacker, Robert W; Diaz, Maria Christina (8 September 2014). "The Porifera Ontology (PORO): enhancing sponge systematics with an anatomy ontology". J Biomed Semantics. 5 (39): 39. doi:10.1186/2041-1480-5-39. PMC 4177528. PMID 25276334.
- Vacelet & Duport 2004, pp. 179–190.
- Bergquist 1978, pp. 183–185.
- Bergquist 1978, pp. 120–127.
- Bergquist 1978, p. 179.
- Clark MA, Choi J and Douglas M (2018) Biology 2e, page 776, OpenStax. ISBN 978-1-947172-52-4.
- Collins AG (December 1998). "Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence". Proceedings of the National Academy of Sciences of the United States of America. 95 (26): 15458–63. Bibcode:1998PNAS...9515458C. doi:10.1073/pnas.95.26.15458. PMC 28064. PMID 9860990.
- Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, et al. (April 2008). "Broad phylogenomic sampling improves resolution of the animal tree of life". Nature. 452 (7188): 745–9. Bibcode:2008Natur.452..745D. doi:10.1038/nature06614. PMID 18322464.
- Hejnol A, Obst M, Stamatakis A, Ott M, Rouse GW, Edgecombe GD, et al. (December 2009). "Assessing the root of bilaterian animals with scalable phylogenomic methods". Proceedings. Biological Sciences. 276 (1677): 4261–70. doi:10.1098/rspb.2009.0896. PMC 2817096. PMID 19759036.
- Ryan JF, Pang K, Schnitzler CE, Nguyen AD, Moreland RT, Simmons DK, et al. (December 2013). "The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution". Science. 342 (6164): 1242592. doi:10.1126/science.1242592. PMC 3920664. PMID 24337300.
- Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, et al. (June 2014). "The ctenophore genome and the evolutionary origins of neural systems". Nature. 510 (7503): 109–14. Bibcode:2014Natur.510..109M. doi:10.1038/nature13400. PMC 4337882. PMID 24847885.
- Pisani, Davide; Pett, Walker; Dohrmann, Martin; Feuda, Roberto; Rota-Stabelli, Omar; Philippe, Hervé; Lartillot, Nicolas; Wörheide, Gert (2015). "Genomic data do not support comb jellies as the sister group to all other animals". Proceedings of the National Academy of Sciences. 112 (50): 15402–15407. Bibcode:2015PNAS..11215402P. doi:10.1073/pnas.1518127112. PMC 4687580. PMID 26621703.
- Berwald, Juli (2017). Spineless: the science of jellyfish and the art of growing a backbone. Riverhead Books.
- Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB (June 2005). "Cultural transmission of tool use in bottlenose dolphins". Proceedings of the National Academy of Sciences of the United States of America. 102 (25): 8939–43. Bibcode:2005PNAS..102.8939K. doi:10.1073/pnas.0500232102. PMC 1157020. PMID 15947077.
- Bergquist 1978, p. 29.
- Bergquist 1978, p. 39.
- Hooper JN, Van Soest RW, Debrenne F (2002). "Phylum Porifera Grant, 1836". In Hooper JN, Van Soest RW (eds.). Systema Porifera: A Guide to the Classification of Sponges. New York: Kluwer Academic/Plenum. pp. 9–14. ISBN 978-0-306-47260-2.
- Ruppert, Fox & Barnes 2004, pp. 76–97
- Bakshani CR, Morales-Garcia AL, Althaus M, Wilcox MD, Pearson JP, Bythell JC, Burgess JG (2018-07-04). "Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection". NPJ Biofilms and Microbiomes. 4 (1): 14. doi:10.1038/s41522-018-0057-2. PMC 6031612. PMID 30002868.
- Bergquist PR (1998). "Porifera". In Anderson DT (ed.). Invertebrate Zoology. Oxford University Press. pp. 10–27. ISBN 978-0-19-551368-4.
- Hinde RT (1998). "The Cnidaria and Ctenophora". In Anderson DT (ed.). Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 978-0-19-551368-4.
- Exposito JY, Cluzel C, Garrone R, Lethias C (November 2002). "Evolution of collagens". The Anatomical Record. 268 (3): 302–16. doi:10.1002/ar.10162. PMID 12382326.
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 82. ISBN 978-0-03-025982-1.
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 82. ISBN 978-0-03-025982-1.
- Rivera AS, Ozturk N, Fahey B, Plachetzki DC, Degnan BM, Sancar A, Oakley TH (April 2012). "Blue-light-receptive cryptochrome is expressed in a sponge eye lacking neurons and opsin". The Journal of Experimental Biology. 215 (Pt 8): 1278–86. doi:10.1242/jeb.067140. PMC 3309880. PMID 22442365.
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 83. ISBN 978-0-03-025982-1. Fig. 5-7
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 83. ISBN 978-0-03-025982-1. Fig. 5-7
- Leys SP (February 2003). "The significance of syncytial tissues for the position of the hexactinellida in the metazoa". Integrative and Comparative Biology. 43 (1): 19–27. doi:10.1093/icb/43.1.19. PMID 21680406.
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 78. ISBN 978-0-03-025982-1.
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 78. ISBN 978-0-03-025982-1.
- Ruppert, Fox & Barnes 2004, p. 83.
- Hickman CP, Roberts LS, Larson A (2001). Integrated Principles of Zoology (11th ed.). New York: McGraw-Hill. p. 247. ISBN 978-0-07-290961-6.
- Halisarca dujardini - Marine Species Identification Portal
- Bergquist PR (2001). "Porifera (Sponges)". Encyclopedia of Life Sciences. John Wiley & Sons, Ltd. doi:10.1038/npg.els.0001582. ISBN 978-0470016176.
- Krautter M (1998). "Ecology of siliceous sponges: Application to the environmental interpretation of the Upper Jurassic sponge facies (Oxfordian) from Spain" (PDF). Cuadernos de Geología Ibérica. 24: 223–239. Archived from the original (PDF) on March 19, 2009. Retrieved 2008-10-10.
- Yahel G, Whitney F, Reiswig HM, Eerkes-Medrano DI, Leys SP (2007). "In situ feeding and metabolism of glass sponges (Hexactinellida, Porifera) studied in a deep temperate fjord with a remotely operated submersible". Limnology and Oceanography. 52 (1): 428–440. Bibcode:2007LimOc..52..428Y. CiteSeerX 10.1.1.597.9627. doi:10.4319/lo.2007.52.1.0428.
- Van Soest, Rob W. M.; Boury-Esnault, Nicole; Vacelet, Jean; Dohrmann, Martin; Erpenbeck, Dirk; De Voogd, Nicole J.; Santodomingo, Nadiezhda; Vanhoorne, Bart; Kelly, Michelle; Hooper, John N. A. (2012). "Global Diversity of Sponges (Porifera)". PLOS One. 7 (4): e35105. Bibcode:2012PLoSO...735105V. doi:10.1371/journal.pone.0035105. PMC 3338747. PMID 22558119.
- "4 new species of 'killer' sponges discovered off Pacific coast". CBC News. April 19, 2014. Archived from the original on April 19, 2014. Retrieved 2014-09-04.
- Vacelet J (2008). "A new genus of carnivorous sponges (Porifera: Poecilosclerida, Cladorhizidae) from the deep N-E Pacific, and remarks on the genus Neocladia" (PDF). Zootaxa. 1752: 57–65. doi:10.11646/zootaxa.1752.1.3. Retrieved 2008-10-31.
- Watling L (2007). "Predation on copepods by an Alaskan cladorhizid sponge". Journal of the Marine Biological Association of the United Kingdom. 87 (6): 1721–1726. doi:10.1017/S0025315407058560.
- Vacelet J, Boury-Esnault N (1995). "Carnivorous sponges". Nature. 373 (6512): 333–335. Bibcode:1995Natur.373..333V. doi:10.1038/373333a0.
- Vacelet J, Kelly M (2008). "New species from the deep Pacific suggest that carnivorous sponges date back to the Early Jurassic". Nature Precedings. doi:10.1038/npre.2008.2327.1.
- Brümmer F, Pfannkuchen M, Baltz A, Hauser T, Thiel V (2008). "Light inside sponges". Journal of Experimental Marine Biology and Ecology. 367 (2): 61–64. doi:10.1016/j.jembe.2008.06.036. Lay summary – BBC News.
- Ruppert, Fox & Barnes 2004, p. 239.
- Ruppert, Fox & Barnes 2004, pp. 90–94.
- Ruppert, Fox & Barnes 2004, pp. 87–88.
- Smith DG, Pennak RW (2001). Pennak's Freshwater Invertebrates of the United States: Porifera to Crustacea (4 ed.). John Wiley and Sons. pp. 47–50. ISBN 978-0-471-35837-4.
- Ruppert, Fox & Barnes 2004, pp. 89–90.
- Ruppert, Fox & Barnes 2004, p. 77.
- Leys SP, Cheung E, Boury-Esnault N (April 2006). "Embryogenesis in the glass sponge Oopsacas minuta: Formation of syncytia by fusion of blastomeres". Integrative and Comparative Biology. 46 (2): 104–17. doi:10.1093/icb/icj016. PMID 21672727.
- Nickel M (December 2004). "Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae)". The Journal of Experimental Biology. 207 (Pt 26): 4515–24. doi:10.1242/jeb.01289. PMID 15579547.
- Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, et al. (June 2007). "A post-synaptic scaffold at the origin of the animal kingdom". PLOS One. 2 (6): e506. Bibcode:2007PLoSO...2..506S. doi:10.1371/journal.pone.0000506. PMC 1876816. PMID 17551586.
- Weaver JC, Aizenberg J, Fantner GE, Kisailus D, Woesz A, Allen P, et al. (April 2007). "Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum". Journal of Structural Biology. 158 (1): 93–106. doi:10.1016/j.jsb.2006.10.027. PMID 17175169.
- Ruzicka R, Gleason DF (January 2008). "Latitudinal variation in spongivorous fishes and the effectiveness of sponge chemical defenses" (PDF). Oecologia. 154 (4): 785–94. Bibcode:2008Oecol.154..785R. doi:10.1007/s00442-007-0874-0. PMID 17960425. Archived from the original (PDF) on 2008-10-06.
- Gage & Tyler 1996, pp. 91–93
- Dunlap M, Pawlik JR (1996). "Video-monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges". Marine Biology. 126 (1): 117–123. doi:10.1007/bf00571383. ISSN 0025-3162.
- Loh TL, Pawlik JR (March 2014). "Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs". Proceedings of the National Academy of Sciences of the United States of America. 111 (11): 4151–6. Bibcode:2014PNAS..111.4151L. doi:10.1073/pnas.1321626111. PMC 3964098. PMID 24567392.
- Piper 2007, p. 148.
- Gochfeld DJ, Easson CG, Slattery M, Thacker RW, Olson JB (2012). Steller D, Lobel L (eds.). "Population Dynamics of a Sponge Disease on Caribbean Reefs". Diving for Science 2012. Proceedings of the American Academy of Underwater Sciences 31st Symposium. Archived from the original on 2015-09-04. Retrieved 2013-11-17.
- Olson JB, Gochfeld DJ, Slattery M (July 2006). "Aplysina red band syndrome: a new threat to Caribbean sponges" (PDF). Diseases of Aquatic Organisms. 71 (2): 163–8. doi:10.3354/dao071163. PMID 16956064. Archived from the original (PDF) on 2016-03-13. Retrieved 2020-01-27. Lay summary – Practical Fishkeeping.
- Wulff JL (June 2008). "Collaboration among sponge species increases sponge diversity and abundance in a seagrass meadow". Marine Ecology. 29 (2): 193–204. Bibcode:2008MarEc..29..193W. doi:10.1111/j.1439-0485.2008.00224.x.
- Duffy JE (1996). "Species boundaries, specialization, and the radiation of sponge-dwelling alpheid shrimp" (PDF). Biological Journal of the Linnean Society. 58 (3): 307–324. doi:10.1111/j.1095-8312.1996.tb01437.x. Archived from the original (PDF) on August 3, 2010.
- Murphy 2002, p. 51.
- Rix, L., de Goeij, J.M., van Oevelen, D., Struck, U., Al-Horani, F.A., Wild, C. and Naumann, M.S. (2018) "Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop". Marine Ecology Progress Series, 589: 85–96. doi:10.3354/meps12443.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C and Naumann MS (2017) "Differential recycling of coral and algal dissolved organic matter via the sponge loop". Funct Ecol, 31: 778−789.
- de Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM and Admiraal W (2013) "Surviving in a marine desert: the sponge loop retains resources within coral reefs". Science, 342: 108−110.
- Rix L, de Goeij JM, Mueller CE, Struck U and others (2016) "Coral mucus fuels the sponge loop in warm- and coldwater coral reef ecosystems". Sci Rep, 6: 18715.
- Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS (2017) "Differential recycling of coral and algal dissolved organic matter via the sponge loop". Funct Ecol 31: 778−789.
- Crossland CJ (1987) In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. Coral Reefs 6: 35−42
- Wild C, Huettel M, Klueter A, Kremb S, Rasheed M, Jorgensen B (2004) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428: 66−70
- Tanaka Y, Miyajima T, Umezawa Y, Hayashibara T, Ogawa H, Koike I (2009) Net release of dissolved organic matter by the scleractinian coral Acropora pulchra. J Exp Mar Biol Ecol 377: 101−106
- Naumann M, Haas A, Struck U, Mayr C, El-Zibdah M, Wild C (2010) Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29: 649−659
- Hoegh-Guldberg O, McCloskey LR, Muscatine L (1987) Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea. Coral Reefs 5: 201−204
- Baghdasarian G, Muscatine L (2000) "Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis". Biol Bull, 199: 278−286
- Crossland CJ, Barnes DJ, Borowitzka MA (1980) "Diurnal lipid and mucus production in the staghorn coral Acropora acuminata". Mar Biol, 60: 81−90.
- Tremblay P, Grover R, Maguer JF, Legendre L, Ferrier-Pagès C (2012) "Autotrophic carbon budget in coral tissue:a new 13C-based model of photosynthate translocation." J Exp Biol, 215: 1384−1393. doi:10.1242/jeb.065201.
- Ferrier-Pagès C, Leclercq N, Jaubert J, Pelegri SP (2000) "Enhancement of pico- and nanoplankton growth by coral exudates". Aquat Microb Ecol, 21: 203−209. doi:10.3354/ame021203.
- Wild C, Niggl W, Naumann MS, Haas AF (2010) "Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O2 availability". Mar Ecol Prog Ser, 411: 61−71. doi:10.3354/meps08653.
- Tanaka Y, Ogawa H, Miyajima T (2011) "Production and bacterial decomposition of dissolved organic matter in a fringing coral reef". J Oceanogr, 67: 427−437. doi:10.1007/s10872-011-0046-z.
- Pita, L., Rix, L., Slaby, B.M., Franke, A. and Hentschel, U. (2018) "The sponge holobiont in a changing ocean: from microbes to ecosystems". Microbiome, 6(1): 46. doi:10.1186/s40168-018-0428-1.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Egan S, Thomas T (2015). "Editorial for: Microbial symbiosis of marine sessile hosts- diversity and function". Frontiers in Microbiology. 6: 585. doi:10.3389/fmicb.2015.00585. PMC 4468920. PMID 26136729.
- Webster NS, Thomas T (April 2016). "The Sponge Hologenome". mBio. 7 (2): e00135-16. doi:10.1128/mBio.00135-16. PMC 4850255. PMID 27103626.
- "Spongia Linnaeus, 1759". World Register of Marine Species. Retrieved 2012-07-18.
- Rowland SM, Stephens T (2001). "Archaeocyatha: A history of phylogenetic interpretation". Journal of Paleontology. 75 (6): 1065–1078. doi:10.1666/0022-3360(2001)075<1065:AAHOPI>2.0.CO;2. JSTOR 1307076. Archived from the original on December 6, 2008.
- Sperling EA, Pisani D, Peterson KJ (January 1, 2007). "Poriferan paraphyly and its implications for Precambrian palaeobiology" (PDF). Geological Society, London, Special Publications. 286 (1): 355–368. Bibcode:2007GSLSP.286..355S. doi:10.1144/SP286.25. Archived from the original (PDF) on May 9, 2009. Retrieved 2012-08-22.
- Whelan NV, Kocot KM, Moroz LL, Halanych KM (May 2015). "Error, signal, and the placement of Ctenophora sister to all other animals". Proceedings of the National Academy of Sciences of the United States of America. 112 (18): 5773–8. Bibcode:2015PNAS..112.5773W. doi:10.1073/pnas.1503453112. PMC 4426464. PMID 25902535.
- Hartman WD, Goreau TF (1970). "Jamaican coralline sponges: Their morphology, ecology and fossil relatives". Symposium of the Zoological Society of London. 25: 205–243. (cited by MGG.rsmas.miami.edu).
- Vacelet J (1985). "Coralline sponges and the evolution of the Porifera". In Conway Morris S, George JD, Gibson R, Platt HM (eds.). The Origins and Relationships of Lower Invertebrates. Oxford University Press. pp. 1–13. ISBN 978-0-19-857181-0.
- Bergquist 1978, pp. 153–154.
- Gazave E, Lapébie P, Renard E, Vacelet J, Rocher C, Ereskovsky AV, Lavrov DV, Borchiellini C (December 2010). "Molecular phylogeny restores the supra-generic subdivision of homoscleromorph sponges (Porifera, Homoscleromorpha)". PLOS One. 5 (12): e14290. Bibcode:2010PLoSO...514290G. doi:10.1371/journal.pone.0014290. PMC 3001884. PMID 21179486.
- Gazave E, Lapébie P, Ereskovsky AV, Vacelet J, Renard E, Cárdenas P, Borchiellini C (May 2012). "No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera" (PDF). Hydrobiologia. 687: 3–10. doi:10.1007/s10750-011-0842-x.
- Cavalcanti FF, Klautau M (2011). "Solenoid: a new aquiferous system to Porifera". Zoomorphology. 130 (4): 255–260. doi:10.1007/s00435-011-0139-7.
- Sperling EA, Robinson JM, Pisani D, Peterson KJ (January 2010). "Where's the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules". Geobiology. 8 (1): 24–36. doi:10.1111/j.1472-4669.2009.00225.x. PMID 19929965.
- Reitner J, Wörheide G (2002). "Non-Lithistid Fossil Demospongiae – Origins of their Palaeobiodiversity and Highlights in History of Preservation". In Hooper JN, Van Soest RW (eds.). Systema Porifera: A Guide to the Classification of Sponges (PDF). New York: Kluwer Academic Plenum. Retrieved November 4, 2008.
- Müller WE, Li J, Schröder HC, Qiao L, Wang X (2007). "The unique skeleton of siliceous sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the Proterozoic: a review". Biogeosciences. 4 (2): 219–232. Bibcode:2007BGeo....4..219M. doi:10.5194/bg-4-219-2007.
- McMenamin MA (2008). "Early Cambrian sponge spicules from the Cerro Clemente and Cerro Rajón, Sonora, México". Geologica Acta. 6 (4): 363–367.
- Li CW, Chen JY, Hua TE (February 1998). "Precambrian sponges with cellular structures". Science. 279 (5352): 879–82. Bibcode:1998Sci...279..879L. doi:10.1126/science.279.5352.879. PMID 9452391.
- "Demospongia". University of California Museum of Paleontology. Archived from the original on October 18, 2013. Retrieved 2008-11-27.
- Vinn O, Wilson MA, Toom U, Mõtus MA (2015). "Earliest known rugosan-stromatoporoid symbiosis from the Llandovery of Estonia (Baltica)". Palaeogeography, Palaeoclimatology, Palaeoecology. 31: 1–5. Bibcode:2015PPP...431....1V. doi:10.1016/j.palaeo.2015.04.023. Retrieved 2015-06-18.
- Kodner RB, Summons RE, Pearson A, King N, Knoll AH (July 2008). "Sterols in a unicellular relative of the metazoans". Proceedings of the National Academy of Sciences of the United States of America. 105 (29): 9897–902. Bibcode:2008PNAS..105.9897K. doi:10.1073/pnas.0803975105. PMC 2481317. PMID 18632573.
- Nichols S, Wörheide G (April 2005). "Sponges: new views of old animals". Integrative and Comparative Biology. 45 (2): 333–4. CiteSeerX 10.1.1.598.4999. doi:10.1093/icb/45.2.333. PMID 21676777.
- Love GD, Grosjean E, Stalvies C, Fike DA, Grotzinger JP, Bradley AS, Kelly AE, Bhatia M, Meredith W, Snape CE, Bowring SA, Condon DJ, Summons RE (February 2009). "Fossil steroids record the appearance of Demospongiae during the Cryogenian period" (PDF). Nature. 457 (7230): 718–21. Bibcode:2009Natur.457..718L. doi:10.1038/nature07673. PMID 19194449. Archived from the original (PDF) on 2018-07-24. Retrieved 2019-08-01.
- Antcliffe JB (2013). Stouge S (ed.). "Questioning the evidence of organic compounds called sponge biomarkers". Palaeontology. 56: 917–925. doi:10.1111/pala.12030.
- Gold DA (Jun 29, 2018). "The slow rise of complex life as revealed through biomarker genetics". Emerging Topics in Life Sciences. 2 (2): 191–199. doi:10.1042/ETLS20170150. PMID 32412622.
- Gold DA, Grabenstatter J, de Mendoza A, Riesgo A, Ruiz-Trillo I, Summons RE (March 2016). "Sterol and genomic analyses validate the sponge biomarker hypothesis". Proceedings of the National Academy of Sciences of the United States of America. 113 (10): 2684–9. Bibcode:2016PNAS..113.2684G. doi:10.1073/pnas.1512614113. PMC 4790988. PMID 26903629.
- Porter SM (2008). "Skeletal microstructure indicates Chancelloriids and Halkieriids are closely related". Palaeontology. 51 (4): 865–879. doi:10.1111/j.1475-4983.2008.00792.x.
- Butterfield NJ, Nicholas CJ (1996). "Burgess Shale-type preservation of both non-mineralizing and "shelly" Cambrian organisms from the Mackenzie Mountains, northwestern Canada". Journal of Paleontology. 70 (6): 893–899. doi:10.1017/S0022336000038579. JSTOR 1306492.
- Janussen D, Steiner M, Zhu MY (2002). "New Well-preserved Scleritomes of Chancelloridae from the Early Cambrian Yuanshan Formation (Chengjiang, China) and the Middle Cambrian Wheeler Shale (Utah, USA) and paleobiological implications". Journal of Paleontology. 76 (4): 596–606. doi:10.1666/0022-3360(2002)076<0596:NWPSOC>2.0.CO;2. Free full text without images at Janussen D (2002). "(as above)". Journal of Paleontology. Archived from the original on December 10, 2008. Retrieved 2008-08-04.
- Borchiellini C, Manuel M, Alivon E, Boury-Esnault N, Vacelet J, Le Parco Y (January 2001). "Sponge paraphyly and the origin of Metazoa". Journal of Evolutionary Biology. 14 (1): 171–179. doi:10.1046/j.1420-9101.2001.00244.x. PMID 29280585.
- Sperling EA, Pisani D, Peterson KJ (2007). "Poriferan paraphyly and its implications for Precambrian paleobiology" (PDF). Journal of the Geological Society of London. 286: 355–368. Bibcode:2007GSLSP.286..355S. doi:10.1144/SP286.25. Archived from the original (PDF) on May 9, 2009. Retrieved 2008-11-04.
- Medina M, Collins AG, Silberman JD, Sogin ML (August 2001). "Evaluating hypotheses of basal animal phylogeny using complete sequences of large and small subunit rRNA". Proceedings of the National Academy of Sciences of the United States of America. 98 (17): 9707–12. Bibcode:2001PNAS...98.9707M. doi:10.1073/pnas.171316998. PMC 55517. PMID 11504944.
- Schierwater B, Eitel M, Jakob W, Osigus HJ, Hadrys H, Dellaporta SL, et al. (January 2009). "Concatenated analysis sheds light on early metazoan evolution and fuels a modern "urmetazoon" hypothesis". PLOS Biology. 7 (1): e20. doi:10.1371/journal.pbio.1000020. PMC 2631068. PMID 19175291.
- Kapli, Paschalia; Telford, Maximilian J. (11 Dec 2020). "Topology-dependent asymmetry in systematic errors affects phylogenetic placement of Ctenophora and Xenacoelomorpha". Science Advances. 6 (10). doi:10.1126/sciadv.abc5162. Retrieved 17 December 2020.
- Smolker RA, Richards AF, Connor RC, Mann J, Berggren P (1997). "Sponge-carrying by Indian Ocean bottlenose dolphins: Possible tool-use by a delphinid". Ethology. 103 (6): 454–465. doi:10.1111/j.1439-0310.1997.tb00160.x. hdl:2027.42/71936.
- Bergquist 1978, p. 88.
- McClenachan L (2008). "Social conflict, Over-fishing and Disease in the Florida Sponge Fishery, 1849–1939". In Starkey DJ, Holm P, Barnard M (eds.). Oceans Past: Management Insights from the History of Marine Animal Populations. Earthscan. pp. 25–27. ISBN 978-1-84407-527-0.
- Jacobson N (2000). Cleavage. Rutgers University Press. p. 62. ISBN 978-0-8135-2715-4.
- "Sponges". Cervical Barrier Advancement Society. 2004. Archived from the original on January 14, 2009. Retrieved 2006-09-17.
- Porterfield WM (1955). "Loofah — The sponge gourd". Economic Botany. 9 (3): 211–223. doi:10.1007/BF02859814.
- Imhoff JF, Stöhr R (2003). "Sponge-Associated Bacteria". In Müller WE (ed.). Sponges (Porifera): Porifera. Springer. pp. 43–44. ISBN 978-3-540-00968-9.
- Teeyapant R, Woerdenbag HJ, Kreis P, Hacker J, Wray V, Witte L, Proksch P (1993). "Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba". Zeitschrift für Naturforschung C. 48 (11–12): 939–45. doi:10.1515/znc-1993-11-1218. PMID 8297426.
- Takeuchi S, Ishibashi M, Kobayashi J, Plakoridine A (1994). "Plakoridine A, a new tyramine-containing pyrrolidine alkaloid from the Okinawan marine sponge Plakortis sp". Journal of Organic Chemistry. 59 (13): 3712–3713. doi:10.1021/jo00092a039.
- Etchells LL, Sardarian A, Whitehead RC (18 April 2005). "A synthetic approach to the plakoridines modeled on a biogenetic theory". Tetrahedron Letters. 46 (16): 2803–2807. doi:10.1016/j.tetlet.2005.02.124.
Further reading
- Bergquist PR (1978). Sponges. London: Hutchinson. ISBN 978-0-520-03658-1.
- Hickman C, Roberts L, Larson A (2003). Animal Diversity (3rd ed.). New York: McGraw-Hill. ISBN 978-0-07-234903-0.
- Ereskovsky AV (2010). The Comparative Embryology of Sponges. Russia: Springer Science+Business Media. ISBN 978-90-481-8575-7.
- Piper R (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Publishing Group. ISBN 978-0-313-33922-6.
- Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7 ed.). Brooks / COLE Publishing. ISBN 978-0-03-025982-1.
- Murphy RC (2002). Coral Reefs: Cities Under The Seas. The Darwin Press, Inc. ISBN 978-0-87850-138-0.
- Gage JD, Tyler PA (1996). Deep-sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge University Press. ISBN 978-0-521-33665-9.
- Vacelet J, Duport E (2004). "Prey capture and digestion in the carnivorous sponge Asbestopluma hypogea (Porifera: Demospongiae)". Zoomorphology. 123 (4): 179–190. doi:10.1007/s00435-004-0100-0.
External links
| Wikimedia Commons has media related to Porifera. |
| Wikispecies has information related to Porifera. |
| The Wikibook Dichotomous Key has a page on the topic of: Porifera |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Sponges. |
- Water flow and feeding in the phylum Porifera (sponges) – Flash animations of sponge body structures, water flow and feeding
- Carsten's Spongepage, Information on the ecology and the biotechnological potential of sponges and their associated bacteria.
- History of [[Tarpon Springs, Florida sponge industry]
- Nature's 'fibre optics' experts
- The Sponge Reef Project
- Queensland Museum information about sponges
- Queensland Museum Sessile marine invertebrates collections
- Queensland Museum Sessile marine invertebrates research
- Sponge Guide for Britain and Ireland, Bernard Picton, Christine Morrow & Rob van Soest
- World Porifera database, the world list of extant sponges, includes a searchable database.
- Sponges: World production and markets // Food and Agriculture Organisation