List of chemical compounds with unusual names
Chemical nomenclature, replete as it is with compounds with complex names, is a repository for some names that may be considered unusual. A browse through the Physical Constants of Organic Compounds in the CRC Handbook of Chemistry and Physics (a fundamental resource) will reveal not just the whimsical work of chemists, but the sometimes peculiar compound names that occur as the consequence of simple juxtaposition. Some names derive legitimately from their chemical makeup, from the geographic region where they may be found, the plant or animal species from which they are isolated or the name of the discoverer.
Some are given intentionally unusual trivial names based on their structure, a notable property or at the whim of those who first isolate them. However, many trivial names predate formal naming conventions. Trivial names can also be ambiguous or carry different meanings in different industries, geographic regions and languages.
Godly noted that "Trivial names having the status of INN or ISO are carefully tailor-made for their field of use and are internationally accepted".[1] In his preface to Chemical Nomenclature, Thurlow wrote that "Chemical names do not have to be deadly serious".[2] A website in existence since 1997[3] and maintained at the University of Bristol lists a selection of "molecules with silly or unusual names" strictly for entertainment. These so-called silly or funny trivial names (of course depending on culture) can also serve an educational purpose. In an article in the Journal of Chemical Education, Dennis Ryan argues that students of organic nomenclature (considered a "dry and boring" subject) may actually take an interest in it when tasked with the job of converting funny-sounding chemical trivial names to their proper systematic names.[4]
The collection listed below presents a sample of trivial names and gives an idea how chemists are inspired when they coin a brand new name for a chemical compound outside of systematic naming. It also includes some examples of systematic names and acronyms that accidentally resemble English words.
Elements
Glenn Seaborg told his students that he proposed the chemical symbol Pu (from P U) instead of the conventional "Pl" for plutonium as a joke, only to find it officially adopted.[5] Unununium (Uuu) was the former temporary name of the chemical element number 111, a synthetic transuranium element. This element was named roentgenium (Rg) in November 2004.
Compounds
Name based on shape
| Barrelene | 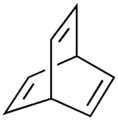 Barrelene |
| Basketane | 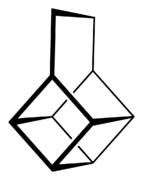 Basketane |
| Cubane | 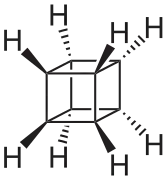 Cubane |
| Dodecahedrane | 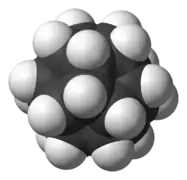 Dodecahedrane |
| Fenestrane | Fenestranes |
| Housane | |
| Ladderane | Pentacycloanammoxic Acid |
| Olympiadane | 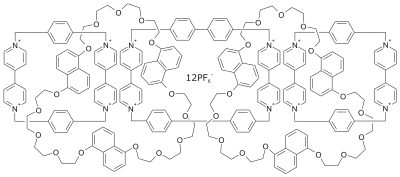 Olympiadane |
| Olympicene | 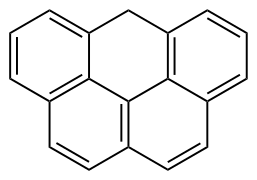 Olympicene |
| Penguinone | 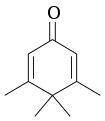 Penguinone |
| Prismane | 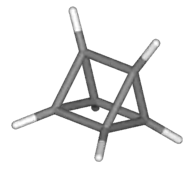 Prismane |
| Quadratic acid |  Squaric acid |
| Sulflower |  Sulflower |
Named after people
| Buckminsterfullerene (Fullerene) | 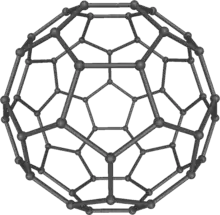 Fullerene |
| Bullvalene | 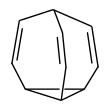 Bullvalene C10H10 |
| Dickite | (Al2Si2O5(OH)4), a clay-like material with a number of manufacturing uses, one of which is as a coating for high-quality bond paper. It is named after its discoverer, Allan Brugh Dick.[14] |
| Josiphos ligands | A well-known catalyst, named after Josi Puleo, the technician who first prepared it.[15] Mandyphos and Taniaphos also exist. |
Named after fictional characters
| Alcindoromycine | An anthracycline antibiotic agent named after the character Alcindoro in La Bohème.[16] |
| Bohemamine | An anti-tumour agent named after the Puccini opera La Bohème.[16] |
| Collinemycin | An anthracycline antibiotic agent named after the character Colline in La Bohème.[16] |
| Ranasmurfin | A blue protein from the foam nests of a tropical frog, named after the Smurfs. |
| Mimimycin | An anthracycline antibiotic agent named after the character Mimì in La Bohème.[16] |
| Musettamycin | An anthracycline antibiotic agent named after the character Musetta in La Bohème.[16] |
| Marcellomycin | An anthracycline antibiotic agent named after the character Marcello in La Bohème.[16] |
| Pikachurin | A retinal protein named after Pokémon character / species Pikachu |
| Rudolphomycin | An anthracycline antibiotic agent named after the character Rodolfo (Rudolph) in La Bohème.[16][17] |
| Sonic hedgehog | A protein named after Sonic the Hedgehog |
Sounding like vulgarisms
| Arsole | 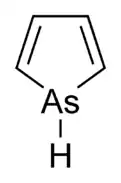 Arsole |
| Bastardane | A close relative to tetramantane (a higher homologue of adamantane), its proper name is nonacyclo[11.7.1.112,18.03,16.04,13.05,10.06,14.07,11.015,20]docosane. Because its unusual ethano-bridge was a deviation from the standard hydrocarbon caged rearrangements, it came to be known as bastardane—the unwanted child.[3][20] |
| Crapinon | An anticholinergic drug, one side effect of which is constipation.[3] |
| Cummingtonite | ((Mg,Fe2+)2(Mg,Fe2+)5Si8O22(OH)2), a magnesium-iron silicate hydroxide, first identified in Cummington, Massachusetts.[3] |
| DAMN | 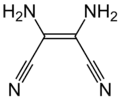 |
| DuPhos | 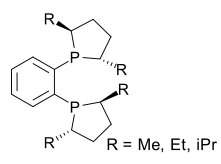 DuPhos |
| Earthcide, or Fartox | Some of the many names for pentachloronitrobenzene, a fungicide.[21] |
| Fucitol | 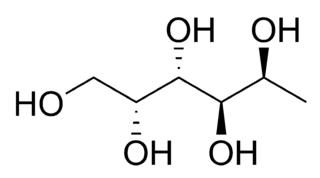 L-Fucitol |
| FucK | The name of the gene that encodes L-fuculokinase, an enzyme that catalyzes a chemical reaction between L-fuculose, ADP, and L-fuculose-1-phosphate.[3] |
| Fukalite | (Ca4Si2O6(CO3)(OH, F))2, a rare form of calcium silicocarbonate discovered in the Fuka Mine of Takahashi, Japan.[3] |
| Pizda |  Pizda |
| Ru(Tris)BiPy-on-a-stick | Shorthand form of (trans-1,4-bis[(4-pyridyl)ethenyl]benzene)(2,2'-bipyridine)ruthenium(II).[22] |
Related to sex
| Fornacite | A rare lead, copper chromate arsenate hydroxide mineral (Pb2CuCrO4AsO4OH), named after its discoverer, Lucien Lewis Forneau.[3] |
| Orotic acid | 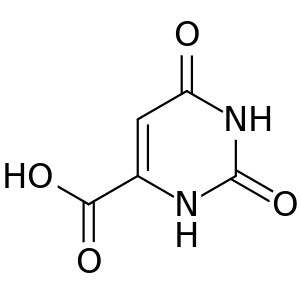 Orotic acid |
| Rhamnetin | 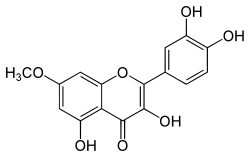 Rhamnetin |
| SEX | An abbreviation of sodium ethyl xanthate,[24] a flotation agent used in the mining industry. |
| Spermine, Spermidine | Spermine Spermidine |
Related to bodily functions
| BARF | 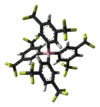 BARF |
| catP | The name of the enzyme responsible for chloramphenicol resistance in various species of bacteria. |
| Constipatic acid |  Constipatic acid |
| dUMP | 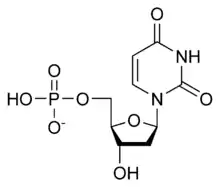 dUMP |
| Nonanal | Nonanal |
| PoO | Chemical formula of polonium monoxide. |
| Uranate | The chemical term for an oxyanion of the element uranium.[3] |
| Vomitoxin | 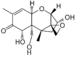 Vomitoxin |
Related to death and decay
| Cadaverine | Cadaverine |
| DEAD, DEADCAT | 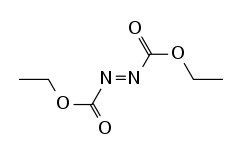 DEAD |
| Putrescine | Putrescine |
Related to religion or legend
| Angelic acid | Angelic acid |
| Diabolic acid | A series of long-chain dicarboxylic acids with chains of different lengths. Named after the Greek word diabollo meaning to mislead.[28] |
| Draculin | 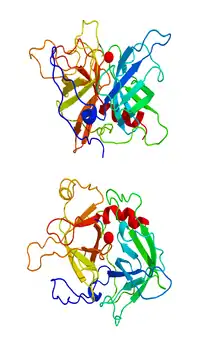 Draculin |
| Luciferase | A generic term for the class of oxidative enzymes that produce bioluminescence. |
| Miraculin | A glycoprotein found in miracle fruit that makes sour foods taste sweet after contact with taste buds.[30] |
Sounds like a name (person, brand or organization)
| Adamantane | Adamantane |
| Irene | Hantzsch-Widman nomenclature for a monocyclic, heterocyclic compound with three ring atoms.[33] |
| Naftazone | (C11H9N3O2), a vasoprotective drug. The NAFTA free-trade zone is the area covered by the North American Free Trade Agreement.[34] |
| PEPPSI | Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation.[35] |
A part sounds like an English word
| Bongkrek acid | 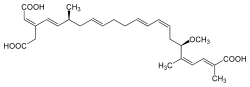 Bongkrek acid |
| Hirsutene |  Hirsutene |
| Magic acid | A superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). |
| Megaphone | A ketone derived from the root of Aniba megaphylla.[38] |
| Moronic acid | 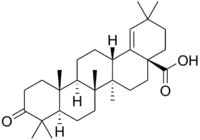 Moronic acid |
| Noggin | A signalling protein involved in embryonic development. |
| Performic acid | A strongly oxidizing acid related to formic acid. |
| Periodic acid | 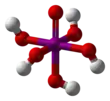 Periodic acid |
| Picket Fence Porphyrin | (5,10,15,20-tetrakis(alpha,alpha,alpha-2-pivalamidophenyl)porphyrin), used to model heme enzyme active sites. |
| Piranha solution | A strongly oxidizing mixture of hydrogen peroxide and sulfuric acid used to remove organic residues from substrates and glassware. The name refers to the voracious appetite of the Amazonian piranha fish. |
| Rednose | A sugar derived from the degradation of rudolphomycin.[16] |
| Rhamnose | 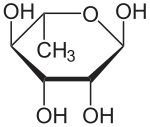 Rhamnose |
| Sillimanite | Aluminium silicate polymorph, sounds like "Silly man-ite" |
| Traumatic acid | Traumatic acid |
Other
| Dinocap | 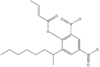 Dinocap |
| Homocubane | 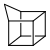 homocubane |
| Methionylthreonylthreonylglutaminylarginyl...isoleucine | The IUPAC name for Titin. This is the largest known protein and so has the longest chemical name. Written in full, it contains 189,819 letters.[39] |
| Periplanone B | 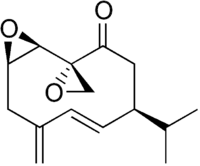 Periplanone B |
| Thebacon | 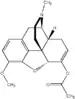 Thebacon |
| FOOF | |
| Gossypol | 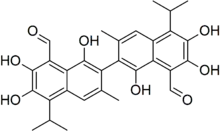 Gossypol |
| Melon | 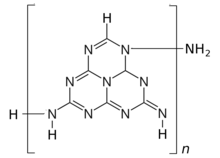 Melon |
See also
Notes and references
- Godly, E.W. (1998). Chemical Nomenclature. Kluwer Academic Publishers. p. 20. ISBN 978-0-7514-0475-3.
- Thurlow, Kevin (1998). Chemical Nomenclature. Kluwer Academic Publishers. xii. ISBN 978-0-7514-0475-3.
- May, Paul (28 May 2013). "Molecules with Silly or Unusual Names". Bristol University. Retrieved 1 November 2013.
- Ryan, Dennis (1997). "Old MacDonald Named a Compound: Branched Enynenynols" (PDF). Journal of Chemical Education. 74 (7): 782. Bibcode:1997JChEd..74..782R. doi:10.1021/ed074p782. Retrieved 2007-08-16.
- Glenn T. Seaborg, Citizen-Scholar, By Peggy House, Reprinted from The Seaborg Center Bulletin, April 1999
- Zimmerman, Howard E.; Robert M. Paufler (1960). "Bicyclo [2.2.2]octa-2,5,7-triene (barrelene), a unique cyclic six electron pi system". Journal of the American Chemical Society. 82 (6): 1514–1515. doi:10.1021/ja01491a071.
- Verbrugge, P. A. (1977). "Unusual organic compounds. XXIV. Compounds with the formula (CH)n. (d). Synthesis of cubane, (CH)8; homocubanes". Chemie en Techniek (Amsterdam). 32 (4): 120–123.
- Pubchem. "Dodecahedrane". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-07-28.
- "'Olympic rings' molecule olympicene in striking image". BBC Online. 27 May 2012. Retrieved 28 May 2012.
- Kroto, H.W.; Heath, J.R.; O'Brien, S.C.; Curl, R.F.; Smalley, R.E. (1985). "C60: Buckminsterfullerene". Nature. 318 (6042): 162. Bibcode:1985Natur.318..162K. doi:10.1038/318162a0. S2CID 4314237.
- Haymet, A.D.J. (1986). "Footballene: a theoretical prediction for the stable, truncated icosahedral molecule C60". J. Am. Chem. Soc. 108 (2): 319. doi:10.1021/ja00262a035.
- Doering, W. von E.; Roth, W. R. (1963). "A Rapidly Reversible Degenerate Cope Rearrangement : Bicyclo[5.1.0]octa-2,5-diene". Tetrahedron. 19 (5): 715–737. doi:10.1016/S0040-4020(01)99207-5.
- Ault, Addison (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". J. Chem. Educ. 78 (7): 924. Bibcode:2001JChEd..78..924A. doi:10.1021/ed078p924.
- Ross, C.; Kerr, P.F. (1931). "Dickite, a Kaolin Mineral" (PDF). American Mineralogist. 15: 34–39.
- Blaser, Hans-Ulrich; Brieden, Walter; Pugin, Benoit; Spindler, Felix; Studer, Martin; Togni, Antonio (2002). "Solvias Josiphos ligands: from discovery to technical applications". Topics in Catalysis. 19 (1): 3–16. doi:10.1023/A:1013832630565. S2CID 95738043.
- Nettleton DE Jr, Balitz DM, Doyle TW, Bradner WT, Johnson DL, O'Herron FA, Schreiber RH, Coon AB, Moseley JE, Myllymaki RW, J Nat Prod. 1980 Mar–Apr;43(2):242–258. DOI: 10.1021/np50008a003
- "Canadian Patents Database CA 1110562: Anthracycline antibiotic designated RUDOLPHOMYCIN". Archived from the original on 2007-11-14. Retrieved 2007-08-16.
- G. Märkl & H. Hauptmann (1983-06-14). "Untersuchungen zur Chemie der Arsole 1,1-dichlor-1-R-λ5-arsole-1-chlorarsole 2,2′,5,5′-tetraphenyldiarsolyl (Studies on the chemistry of arsoles)". J. Organomet. Chem. 248 (3): 269–285. doi:10.1016/S0022-328X(00)98709-6.
- Mikael P. Johansson & Jonas Jusélius (2005). "Arsole Aromaticity Revisited". Lett. Org. Chem. (3): 469–474.
- Schleyer, Paul von Rague; Eiji Osawa; Michael G. B. Drew (1968). "Nonacyclo[11.7.1.12,18.03,16.04,13.05,10.06,14.07,11.015,20]docosane, a bastard tetramantane" (PDF). J. Am. Chem. Soc. 90 (18): 5034–5036. doi:10.1021/ja01020a053. Retrieved 2007-08-15.
- "NIST Standard Reference Database 69, June 2005 Release: NIST Chemistry WebBook – Pentachloronitrobenzene". Retrieved 2007-08-16.
- Toma, SH; Uemi, M; Nikolaou, S; Tomazela, DM; Eberlin, MN; Toma, HE (2004). "{trans-1,4-Bis[(4-pyridyl)ethenyl]benzene}(2,2'-bipyridine)ruthenium(II) Complexes and Their Supramolecular Assemblies with β-Cyclodextrin". Inorg Chem. 43 (11): 3521–3527. doi:10.1021/ic0352250. PMID 15154817.
- Uri J, Csoban G, Viragh E., Acta Physiol Hung. 1951;2(2):223-8.
- See, for example, Okibe, N; Johnson, DB (2004). "Toxicity of flotation reagents to moderately thermophilic bioleaching microorganisms". Biotechnology Letters. 24 (23): 2011–2016. doi:10.1023/A:1021118915720. S2CID 23948075.
- "BARF". ChemSpider. Royal Society of Chemistry. 2013. Retrieved 4 November 2013.
- Chester, DO (1979). "Three New Aliphatic Acids from Lichens of Genus Parmelia (Subgenus Xanthoparmelia )". Australian Journal of Chemistry. 32 (11): 2565. doi:10.1071/CH9792565.
- Nordenström, Björn E. W. (1951). "Effect of cadaverine and lysine on the urinary excretion of piperidine in rabbits". Acta Pharmacologica et Toxicologica. 7 (3): 287–296. doi:10.1111/j.1600-0773.1951.tb02870.x. PMID 14856760.
- R A Klein, G P Hazlewood, P Kemp, and R M Dawson, Biochem J. 1979 December 1; 183(3): 691–700.
- Apitz-Castro R, Béguin S, Tablante A, Bartoli F, Holt JC, Hemker HC (1995). "Purification and partial characterization of draculin, the anticoagulant factor present in the saliva of vampire bats (Desmodus rotundus)". Thromb. Haemost. 73 (1): 94–100. doi:10.1055/s-0038-1653731. PMID 7740503.
- Theerasilp S, Kurihara Y (August 1988). "Complete purification and characterization of the taste-modifying protein, miraculin, from miracle fruit". J. Biol. Chem. 263 (23): 11536–11539. PMID 3403544.
- Prelog, V., Seiwerth, R. (1941). "Über eine neue, ergiebigere Darstellung des Adamantans". Berichte. 74 (11): 1769–1772. doi:10.1002/cber.19410741109.CS1 maint: multiple names: authors list (link)
- Not to be confused with the fictional material adamantium
- Parent Hydride Names and Substantive Nomenclature (PDF). IUPAC. March 2004. p. 16.
- Charles O, Coolsaet B (1972). "[Prevention of hemorrhage in prostatic surgery. Apropos of the study of the hemostatic activity in prostatectomy of a new molecule: beta-naphthoquinone monosemicarbazone (Naftazone)]". Annales d'Urologie (in French). 6 (3): 209–212. PMID 4562066.
- "PEPPSI Catalysts". Retrieved 2009-04-01.
- Curran, Dennis P. (1985). "Tandem radical approach to linear condensed cyclopentanoids. Total synthesis of (.+-.)-hirsutene". Journal of the American Chemical Society. 107 (5): 1448–1449. doi:10.1021/ja00291a077.
- Nozoe, Shigeo (1976). "Isolation, structure and synthesis of hirsutene, a precursor hydrocarbon of coriolin biosynthesis". Tetrahedron Letters. 17 (3): 195–198. doi:10.1016/0040-4039(76)80013-5.
- SM Kupchan, KL Stevens, EA Rohlfing, BR Sickles, AT Sneden, RW Miller, RF Bryan, J. Org. Chem., 43(4) (1978) 586
- Sam Kean (2011), The Disappearing Spoon, Little, Brown, p. 36, ISBN 9781446437650
- Derek, Lowe (2010-02-23). "Things I Won't Work With: Dioxygen Difluoride". In the Pipeline. Retrieved 2019-04-02.
Bibliography
- E.C. Alyea, "Metal Complexes of Ditertiary Arsines. Chapter in Transition Metal Complexes of Phosphorus, Arsenic and Antimony Ligands", MacMillan, 1973. Chapter: "Some amusing names of arsine ligands: edas, vdias, dam, ffars etc"
- J. Andraos, "Glossary of Coined Names & Terms Used in Science", York University, 2004.
- Giles, P.M. (1999). "Revised Section F: Natural products and related compounds". Pure Appl. Chem. 71 (4): 587–643. doi:10.1351/pac199971040587. S2CID 94379874. Retrieved 2007-08-16.
- Aronson, Jeff (1999). "That's show business". British Medical Journal. 319 (7215): 972. doi:10.1136/bmj.319.7215.972. PMC 1116803. PMID 10514162.
- Browne, Malcolm W. (April 22, 1986). "Chemists dabble in whimsy". International New York Times. Retrieved 3 October 2013.
- Paul May, "Molecules with Silly or Unusual Names," Imperial College Press, July 2008, ISBN 978-1-84816-207-5.
- Metanomski, W. V. (1987). "Unusual Names Assigned to chemical substances". Chem. Int. 9: 211–215.
- Monk, Felonious (13 April 2006). "Chemical Cock-ups: A Story of How Not to Name a Chemical Compound". h2g2: The guide to life, the universe and everything. Not Panicking Ltd. Retrieved 30 October 2013.
- Alex Nickon and Ernest F. Silversmith, "Organic Chemistry, the Name Game: Modern Coined Terms and Their Origins", Pergamon 1987. ISBN 0-08-034481-X.
- Randall, David (February 1, 2004). "Storyville: Molecular scientists have a word for it". The Independent on Sunday. London, England.(subscription required)
- Wallechinsky, David; Wallace, Amy (2005). "24. Molecules and amoebas with funny names". The new book of lists : the original compendium of curious information. New York, N.Y.: Canongate. pp. 203–205. ISBN 9781841957197.