Lysergic acid diethylamide
Lysergic acid diethylamide (LSD),[lower-alpha 1] also known colloquially as acid, is a hallucinogenic drug.[11] Effects typically include altered thoughts, feelings, and awareness of one's surroundings.[11] Many users have visual or auditory hallucinations.[12][13] Dilated pupils, increased blood pressure, and increased body temperature are typical.[14] Effects typically begin within half an hour and can last for up to 20 hours.[14][15] It is used mainly as a recreational drug or for spiritual reasons.[14][16]
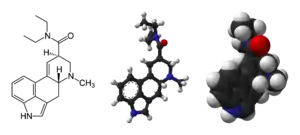 2D structural formula and 3D models of LSD | |
| Clinical data | |
|---|---|
| Pronunciation | /daɪ eθəl ˈæmaɪd/, /æmɪd/, or /eɪmaɪd/[1][2][3] |
| Other names | LSD, LSD-25, Acid, Delysid, others |
| AHFS/Drugs.com | Reference |
| Dependence liability | Low[4] |
| Addiction liability | Low-rare[5] |
| Routes of administration | By mouth, under the tongue, intravenous |
| Drug class | Hallucinogen (serotonergic psychedelic) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 71%[6] |
| Protein binding | Unknown[7] |
| Metabolism | Liver (CYP450)[6] |
| Metabolites | 2-Oxo-3-hydroxy-LSD[6] |
| Onset of action | 30–40 minutes[8] |
| Elimination half-life | 3.6 hours[6][9] |
| Duration of action | 8–20 hours[10] |
| Excretion | Kidneys[6][9] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.031 |
| Chemical and physical data | |
| Formula | C20H25N3O |
| Molar mass | 323.440 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 80 to 85 °C (176 to 185 °F) |
| |
| |
| (verify) | |
LSD is not physiologically addictive, but its frequent use can lead to psychological addiction.[11][17] Adverse psychiatric reactions are possible, such as anxiety, paranoia, and delusions.[7] Distressing flashbacks might occur in spite of no further use, a condition called hallucinogen persisting perception disorder.[18][19] Death as a result of LSD overdose is virtually unknown; on extremely rare occasions, however, death can be the result of accidents or reckless behavior.[14] The effects of LSD are believed to occur as a result of alterations in the serotonin system.[14] As little as 20 micrograms can produce a noticeable effect.[14] In pure form, LSD is clear or white in color, has no smell, and is crystalline.[11] It breaks down with exposure to ultraviolet light.[14]
As of 2017, about 10 percent of people in the United States have used LSD at some point in their lives, while 0.7 percent have used it in the last year.[20] It was most popular in the 1960s to 1980s.[14] The use of LSD among US adults increased 56.4% from 2015 to 2018.[21] LSD is typically either swallowed or held under the tongue.[11] It is most often sold on blotter paper and less commonly as tablets or in gelatin squares.[14]
LSD was first made by Albert Hofmann in 1938 from lysergic acid, a chemical from a fungus that grows ergot.[14][18] Hofmann discovered its hallucinogenic properties in 1943.[22] In the 1950s, the Central Intelligence Agency (CIA) believed that the drug might be useful for mind control, so they tested it on people, some without their knowledge, in a program called MKUltra.[23] LSD was sold as a medication for research purposes under the trade-name Delysid in the 1950s and 1960s.[14][24] It was listed as a schedule 1 controlled substance by the United Nations in 1971.[14] It currently has no approved medical use.[14] In Europe, as of 2011, the typical cost of a dose was between €4.50 and €25.[14] In November 2020, a referendum determined that it would be decriminalized in the US state of Oregon.[25]
Uses
Recreational
LSD is commonly used as a recreational drug in the company of friends, in large crowds, or by oneself.[26] The street price of a single dose of LSD can be anywhere from $2 to $50.[27]
Spiritual
LSD can catalyze intense spiritual experiences and is thus considered an entheogen. Some user have reported out of body experiences. In 1966, Timothy Leary established the League for Spiritual Discovery with LSD as its sacrament.[28][29] Stanislav Grof has written that religious and mystical experiences observed during LSD sessions appear to be phenomenologically indistinguishable from similar descriptions in the sacred scriptures of the great religions of the world and the texts of ancient civilizations.[30]
Effects
Physical
LSD can cause pupil dilation, reduced appetite, profuse sweating, and wakefulness. Other physical reactions to LSD are highly variable and nonspecific, some of which may be secondary to the psychological effects of LSD. Among the reported symptoms are elevated body temperature, blood sugar, and heart rate, alongside goose bumps, jaw clenching, mouth dryness, and hyperreflexia. In negative experiences, numbness, weakness, nausea, and tremors have also been exhibited.[14]
Psychological
The most common immediate psychological effects of LSD are visual hallucinations and illusions (colloquially known as "trips"), which vary depending on how much is used and how the dosage interacts with the brain. Trips usually start within 20–30 minutes of taking LSD orally (less if snorted or taken intravenously), peak three to four hours after ingestion, and last up to 20 hours. Good trips are reportedly deeply stimulating and pleasurable, and typically involve intense joy or euphoria, a greater appreciation for life, reduced anxiety, a sense of spiritual enlightenment, and a sense of belonging or interconnectedness with the universe.[37][38] Negative experiences, colloquially known as "bad trips," evoke an array of dark emotions, such as irrational fear, anxiety, panic, paranoia, dread, distrustfulness, hopelessness, intrusive thoughts of harming others, and even suicidal ideation.[39] While it is impossible to predict when a bad trip will occur, one's mood, surroundings, sleep, hydration, social setting, and other factors can be controlled to minimize the risk of a bad trip.[40][41]
Sensory
LSD causes an animated sensory experience of senses, emotions, memories, time, and awareness for 6 to 20 hours, depending on dosage and tolerance.[15] Generally beginning within 30 to 90 minutes after ingestion, the user may experience anything from subtle changes in perception to overwhelming cognitive shifts. Changes in auditory and visual perception are typical.[42][43]
Some sensory effects may include an experience of radiant colors, objects and surfaces appearing to ripple or "breathe," spinning fractals superimposed on one's vision, colored patterns behind the closed eyelids, an altered sense of time, geometric patterns emerging on walls and other textured objects, and morphing objects.[42] Some users report a strong metallic taste for the duration of the effects.[44]
Some report that the inanimate world appears to animate in an inexplicable way; for instance, objects that are static in three dimensions can seem to be moving relative to one or more additional spatial dimensions.[45] Many of the basic visual effects resemble the phosphenes seen after applying pressure to the eye and have also been studied as form constants. The auditory effects of LSD may include echo-like distortions of sounds, changes in ability to discern concurrent auditory and visual stimuli, and a general intensification of the experience of music. Higher doses often cause intense and fundamental distortions of sensory perception such as synesthesia, the experience of additional spatial or temporal dimensions, and temporary dissociation.
Adverse effects
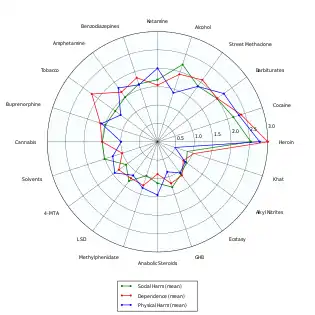
Out of the 20 drugs ranked in order of individual and societal harm by David Nutt, LSD was third to last, or approximately 1/10th as harmful as alcohol. The most significant adverse effect of LSD was impairment of mental functioning while intoxicated.[47]
Mental disorders
LSD may trigger panic attacks or feelings of extreme anxiety, known familiarly as a "bad trip." Review studies suggest that LSD likely plays a role in precipitating the onset of acute psychosis in previously healthy individuals with an increased likelihood in individuals who have a family history of schizophrenia.[7][48] There is evidence that people with severe mental illnesses like schizophrenia have a higher likelihood of experiencing adverse effects from taking LSD.[48]
Suggestibility
While publicly available documents indicate that the CIA and Department of Defense have discontinued research into the use of LSD as a means of mind control,[49] research from the 1960s suggests that both mentally ill and healthy people are more suggestible while under its influence.[50][51][52]
Flashbacks
"Flashbacks" are a reported psychological phenomenon in which an individual experiences an episode of some of LSD's subjective effects after the drug has worn off, persisting for months or years after hallucinogen use.[53] Individuals with hallucinogen persisting perception disorder experience intermittent or chronic flashbacks that cause distress or impairment in life and work.[19]
Cancer and pregnancy
The mutagenic potential of LSD is unclear. Overall, the evidence seems to point to limited or no effect at commonly used doses.[54] Studies showed no evidence of teratogenic or mutagenic effects.[7]
Addiction and tolerance
Tolerance to LSD builds up with consistent use[55] and cross-tolerance has been demonstrated between LSD, mescaline,[56] and psilocybin.[57] Researchers believe that tolerance returns to baseline after two weeks of being drug-free.[58]
The NIH states that LSD is addictive,[18] while most other sources state it is not.[17][59] A 2009 textbook states that it "rarely produce[s] compulsive use."[5] A 2006 review states it is readily abused but does not result in addiction.[17]
Overdose
As of 2008, there were no documented fatalities attributed to an LSD overdose.[7] Despite this, several behavioral-related fatalities and suicides have occurred due to LSD.[60][61] Eight individuals who accidentally consumed very high amounts by mistaking LSD for cocaine developed comatose states, hyperthermia, vomiting, gastric bleeding, and respiratory problems—all survived, however, with supportive care.[7]
Reassurance in a calm, safe environment is beneficial. Agitation can be safely addressed with benzodiazepines such as lorazepam or diazepam. Neuroleptics such as haloperidol are recommended against because they may have adverse effects. LSD is rapidly absorbed, so activated charcoal and emptying of the stomach is of little benefit, unless done within 30–60 minutes of ingesting an overdose of LSD. Sedation or physical restraint is rarely required, and excessive restraint may cause complications such as hyperthermia (over-heating) or rhabdomyolysis.[62]
Research suggests that massive doses are not lethal, but do typically require supportive care, which may include endotracheal intubation or respiratory support.[62] It is recommended that high blood pressure, tachycardia (rapid heart-beat), and hyperthermia, if present, are treated symptomatically, and that low blood pressure is treated initially with fluids and then with pressors if necessary. Intravenous administration of anticoagulants, vasodilators, and sympatholytics may be useful with massive doses.[62]
Pharmacology
Pharmacodynamics
Most serotonergic psychedelics are not significantly dopaminergic, and LSD is therefore atypical in this regard. The agonism of the D2 receptor by LSD may contribute to its psychoactive effects in humans.[63][64]
LSD binds to most serotonin receptor subtypes except for the 5-HT3 and 5-HT4 receptors. However, most of these receptors are affected at too low affinity to be sufficiently activated by the brain concentration of approximately 10–20 nM.[59] In humans, recreational doses of LSD can affect 5-HT1A (Ki=1.1nM), 5-HT2A (Ki=2.9nM), 5-HT2B (Ki=4.9nM), 5-HT2C (Ki=23nM), 5-HT5A (Ki=9nM [in cloned rat tissues]), and 5-HT6 receptors (Ki=2.3nM).[65][66] Although not present in humans, 5-HT5B receptors found in rodents also have a high affinity for LSD.[67] The psychedelic effects of LSD are attributed to cross-activation of 5-HT2A receptor heteromers.[68] Many but not all 5-HT2A agonists are psychedelics and 5-HT2A antagonists block the psychedelic activity of LSD. LSD exhibits functional selectivity at the 5-HT2A and 5HT2C receptors in that it activates the signal transduction enzyme phospholipase A2 instead of activating the enzyme phospholipase C as the endogenous ligand serotonin does.[69]
Exactly how LSD produces its effects is unknown, but it is thought that it works by increasing glutamate release in the cerebral cortex[59] and therefore excitation in this area, specifically in layers IV and V.[70] LSD, like many other drugs of recreational use, has been shown to activate DARPP-32-related pathways.[71] The drug enhances dopamine D2 receptor protomer recognition and signaling of D2–5-HT2A receptor complexes,[72] which may contribute to its psychotic effects.[72] LSD has been shown to have low affinity for H1 receptors, displaying antihistamine effects.[73][74][75]
The crystal structure of LSD bound in its active state to a serotonin receptor, specifically the 5-HT2B receptor, has been elucidated for the first time in 2017.[76][77][78] The LSD-bound 5-HT2B receptor is regarded as an excellent model system for the 5-HT2A receptor and the structure of the LSD-bound 5-HT2B receptor was used in the study as a template to determine the structural features necessary for the activity of LSD at the 5-HT2A receptor.[76][77][78] The diethylamide moiety of LSD was found to be a key component for its activity, which is in accordance with the fact that the related lysergamide lysergic acid amide (LSA) is far less hallucinogenic in comparison.[78] LSD was found to stay bound to both the 5-HT2A and 5-HT2B receptors for an exceptionally long amount of time, which may be responsible for its long duration of action in spite of its relatively short terminal half-life.[76][77][78] The extracellular loop 2 leucine 209 residue of the 5-HT2B receptor forms a 'lid' over LSD that appears to trap it in the receptor, and this was implicated in the potency and functional selectivity of LSD and its very slow dissociation rate from the 5-HT2 receptors.[76][77][78]
Pharmacokinetics
The effects of LSD normally last between 6 and 12 hours depending on dosage, tolerance, body weight, and age.[79] The Sandoz prospectus for "Delysid" warned: "intermittent disturbances of affect may occasionally persist for several days."[80] Aghajanian and Bing (1964) found LSD had an elimination half-life of only 175 minutes (about 3 hours).[65] However, using more accurate techniques, Papac and Foltz (1990) reported that 1 µg/kg oral LSD given to a single male volunteer had an apparent plasma half-life of 5.1 hours, with a peak plasma concentration of 5 ng/mL at 3 hours post-dose.[81]
The pharmacokinetics of LSD were not properly determined until 2015, which is not surprising for a drug with the kind of low-μg potency that LSD possesses.[9][6] In a sample of 16 healthy subjects, a single mid-range 200 μg oral dose of LSD was found to produce mean maximal concentrations of 4.5 ng/mL at a median of 1.5 hours (range 0.5–4 hours) post-administration.[9][6] After attainment of peak levels, concentrations of LSD decreased following first-order kinetics with a terminal half-life of 3.6 hours for up to 12 hours and then with slower elimination with a terminal half-life of 8.9 hours thereafter.[9][6] The effects of the dose of LSD given lasted for up to 12 hours and were closely correlated with the concentrations of LSD present in circulation over time, with no acute tolerance observed.[9][6] Only 1% of the drug was eliminated in urine unchanged whereas 13% was eliminated as the major metabolite 2-oxo-3-hydroxy-LSD (O-H-LSD) within 24 hours.[9][6] O-H-LSD is formed by cytochrome P450 enzymes, although the specific enzymes involved are unknown, and it does not appear to be known whether O-H-LSD is pharmacologically active or not.[9][6] The oral bioavailability of LSD was crudely estimated as approximately 71% using previous data on intravenous administration of LSD.[9][6] The sample was equally divided between male and female subjects and there were no significant sex differences observed in the pharmacokinetics of LSD.[9][6]
Chemistry

LSD is a chiral compound with two stereocenters at the carbon atoms C-5 and C-8, so that theoretically four different optical isomers of LSD could exist. LSD, also called (+)-D-LSD, has the absolute configuration (5R,8R). The C-5 isomers of lysergamides do not exist in nature and are not formed during the synthesis from d-lysergic acid. Retrosynthetically, the C-5 stereocenter could be analysed as having the same configuration of the alpha carbon of the naturally occurring amino acid L-tryptophan, the precursor to all biosynthetic ergoline compounds.
However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of bases, as the alpha proton is acidic and can be deprotonated and reprotonated. Non-psychoactive iso-LSD which has formed during the synthesis can be separated by chromatography and can be isomerized to LSD.
Pure salts of LSD are triboluminescent, emitting small flashes of white light when shaken in the dark.[79] LSD is strongly fluorescent and will glow bluish-white under UV light.
Synthesis
LSD is an ergoline derivative. It is commonly synthesized by reacting diethylamine with an activated form of lysergic acid. Activating reagents include phosphoryl chloride[82] and peptide coupling reagents.[83] Lysergic acid is made by alkaline hydrolysis of lysergamides like ergotamine, a substance usually derived from the ergot fungus on agar plate; or, theoretically possible, but impractical and uncommon, from ergine (lysergic acid amide, LSA) extracted from morning glory seeds.[84] Lysergic acid can also be produced synthetically, eliminating the need for ergotamines.[85][86]
Dosage

A single dose of LSD may be between 40 and 500 micrograms—an amount roughly equal to one-tenth the mass of a grain of sand. Threshold effects can be felt with as little as 25 micrograms of LSD.[87][88] Dosages of LSD are measured in micrograms (µg), or millionths of a gram. By comparison, dosages of most drugs, both recreational and medicinal, are measured in milligrams (mg), or thousandths of a gram. For example, an active dose of mescaline, roughly 0.2 to 0.5 g, has effects comparable to 100 µg or less of LSD.[80]
In the mid-1960s, the most important black market LSD manufacturer (Owsley Stanley) distributed acid at a standard concentration of 270 µg,[89] while street samples of the 1970s contained 30 to 300 µg. By the 1980s, the amount had reduced to between 100 and 125 µg, dropping more in the 1990s to the 20–80 µg range,[90] and even more in the 2000s (decade).[89][91]
Reactivity and degradation
"LSD," writes the chemist Alexander Shulgin, "is an unusually fragile molecule ... As a salt, in water, cold, and free from air and light exposure, it is stable indefinitely."[79]
LSD has two labile protons at the tertiary stereogenic C5 and C8 positions, rendering these centres prone to epimerisation. The C8 proton is more labile due to the electron-withdrawing carboxamide attachment, but removal of the chiral proton at the C5 position (which was once also an alpha proton of the parent molecule tryptophan) is assisted by the inductively withdrawing nitrogen and pi electron delocalisation with the indole ring.
LSD also has enamine-type reactivity because of the electron-donating effects of the indole ring. Because of this, chlorine destroys LSD molecules on contact; even though chlorinated tap water contains only a slight amount of chlorine, the small quantity of compound typical to an LSD solution will likely be eliminated when dissolved in tap water.[79] The double bond between the 8-position and the aromatic ring, being conjugated with the indole ring, is susceptible to nucleophilic attacks by water or alcohol, especially in the presence of light. LSD often converts to "lumi-LSD," which is inactive in human beings.[79]
A controlled study was undertaken to determine the stability of LSD in pooled urine samples.[92] The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 °C for up to four weeks. After four weeks of incubation, a 30% loss in LSD concentration at 37 °C and up to a 40% at 45 °C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. Stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of EDTA.
Detection
LSD may be quantified in urine as part of a drug abuse testing program, in plasma or serum to confirm a diagnosis of poisoning in hospitalized victims or in whole blood to assist in a forensic investigation of a traffic or other criminal violation or a case of sudden death. Both the parent drug and its major metabolite are unstable in biofluids when exposed to light, heat or alkaline conditions and therefore specimens are protected from light, stored at the lowest possible temperature and analyzed quickly to minimize losses.[93]
The apparent plasma half life of LSD is considered to be around 5.1 hours with peak plasma concentrations occurring 3 hours after administration.[94]
LSD can be detected using an Ehrlich's reagent and a Hofmann's reagent.
History
—Albert Hofmann, on his first experience with LSD[95]
LSD was first synthesized on November 16, 1938[96] by Swiss chemist Albert Hofmann at the Sandoz Laboratories in Basel, Switzerland as part of a large research program searching for medically useful ergot alkaloid derivatives. LSD's psychedelic properties were discovered 5 years later when Hofmann himself accidentally ingested an unknown quantity of the chemical.[97] The first intentional ingestion of LSD occurred on April 19, 1943,[98] when Hofmann ingested 250 µg of LSD. He said this would be a threshold dose based on the dosages of other ergot alkaloids. Hofmann found the effects to be much stronger than he anticipated.[99] Sandoz Laboratories introduced LSD as a psychiatric drug in 1947 and marketed LSD as a psychiatric panacea, hailing it "as a cure for everything from schizophrenia to criminal behavior, 'sexual perversions,' and alcoholism."[100] The abbreviation "LSD" is from the German "Lysergsäurediethylamid".[101]

Beginning in the 1950s, the US Central Intelligence Agency (CIA) began a research program code named Project MKUltra. The CIA introduced LSD to the United States, purchasing the entire world's supply for $240,000 and propagating the LSD, through CIA front organizations to American hospitals, clinics, prisons and research centers.[102] Experiments included administering LSD to CIA employees, military personnel, doctors, other government agents, prostitutes, mentally ill patients, and members of the general public in order to study their reactions, usually without the subjects' knowledge. The project was revealed in the US congressional Rockefeller Commission report in 1975.
In 1963, the Sandoz patents expired on LSD.[90] Several figures, including Aldous Huxley, Timothy Leary, and Al Hubbard, began to advocate the consumption of LSD. LSD became central to the counterculture of the 1960s.[103] In the early 1960s the use of LSD and other hallucinogens was advocated by new proponents of consciousness expansion such as Leary, Huxley, Alan Watts and Arthur Koestler,[104][105] and according to L. R. Veysey they profoundly influenced the thinking of the new generation of youth.[106]
On October 24, 1968, possession of LSD was made illegal in the United States.[107] The last FDA approved study of LSD in patients ended in 1980, while a study in healthy volunteers was made in the late 1980s. Legally approved and regulated psychiatric use of LSD continued in Switzerland until 1993.[108]
In November 2020, Oregon became the first US state to decriminalize possession of small amounts of LSD after voters approved Ballot Measure 110.[109]
Society and culture
Counterculture

By the mid-1960s, the youth countercultures in California, particularly in San Francisco, had adopted the use of hallucinogenic drugs, with the first major underground LSD factory established by Owsley Stanley.[110] From 1964, the Merry Pranksters, a loose group that developed around novelist Ken Kesey, sponsored the Acid Tests, a series of events primarily staged in or near San Francisco, involving the taking of LSD (supplied by Stanley), accompanied by light shows, film projection and discordant, improvised music known as the psychedelic symphony.[111][112] The Pranksters helped popularize LSD use, through their road trips across America in a psychedelically-decorated converted school bus, which involved distributing the drug and meeting with major figures of the beat movement, and through publications about their activities such as Tom Wolfe's The Electric Kool-Aid Acid Test (1968).[113]
In San Francisco's Haight-Ashbury neighborhood, brothers Ron and Jay Thelin opened the Psychedelic Shop in January 1966.[114] The Thelins opened the store to promote safe use of LSD, which was then still legal in California. The Psychedelic Shop helped to further popularize LSD in the Haight and to make the neighborhood the unofficial capital of the hippie counterculture in the United States. Ron Thelin was also involved in organizing the Love Pageant rally, a protest held in Golden Gate park to protest California's newly adopted ban on LSD in October 1966. At the rally, hundreds of attendees took acid in unison. Although the Psychedelic Shop closed after barely a year-and-a-half in business, its role in popularizing LSD was considerable.[115]
A similar and connected nexus of LSD use in the creative arts developed around the same time in London. A key figure in this phenomenon in the UK was British academic Michael Hollingshead, who first tried LSD in America in 1961 while he was the Executive Secretary for the Institute of British-American Cultural Exchange. After being given a large quantity of pure Sandoz LSD (which was still legal at the time) and experiencing his first "trip," Hollingshead contacted Aldous Huxley, who suggested that he get in touch with Harvard academic Timothy Leary, and over the next few years, in concert with Leary and Richard Alpert, Hollingshead played a major role in their famous LSD research at Millbrook before moving to New York City, where he conducted his own LSD experiments. In 1965 Hollingshead returned to the UK and founded the World Psychedelic Center in Chelsea, London.
Music and art
In both music and art, the influence of LSD was soon being more widely seen and heard thanks to the bands that participated in the Acid Tests and related events, including the Grateful Dead, Jefferson Airplane and Big Brother and the Holding Company, and through the inventive poster and album art of San Francisco-based artists like Rick Griffin, Victor Moscoso, Bonnie MacLean, Stanley Mouse & Alton Kelley, and Wes Wilson, meant to evoke the visual experience of an LSD trip. LSD had a strong influence on the Grateful Dead and the culture of "Deadheads."[116]
Among the many famous people in the UK that Michael Hollingshead is reputed to have introduced to LSD are artist and Hipgnosis founder Storm Thorgerson, and musicians Donovan, Keith Richards, Paul McCartney, John Lennon, and George Harrison. Although establishment concern about the new drug led to it being declared an illegal drug by the Home Secretary in 1966, LSD was soon being used widely in the upper echelons of the British art and music scene, including members of the Beatles, the Rolling Stones, the Moody Blues, the Small Faces, Pink Floyd, Jimi Hendrix and others, and the products of these experiences were soon being both heard and seen by the public with singles like the Small Faces' "Itchycoo Park" and LPs like the Beatles' Sgt. Pepper's Lonely Hearts Club Band and Cream's Disraeli Gears, which featured music that showed the obvious influence of the musicians' recent psychedelic excursions, and which were packaged in elaborately-designed album covers that featured vividly-coloured psychedelic artwork by artists like Peter Blake, Martin Sharp, Hapshash and the Coloured Coat (Nigel Waymouth and Michael English) and art/music collective The Fool.
In the 1960s, musicians from psychedelic music and psychedelic rock bands began to refer (at first indirectly, and later explicitly) to the drug and attempted to recreate or reflect the experience of taking LSD in their music. A number of features are often included in psychedelic music. Exotic instrumentation, with a particular fondness for the sitar and tabla are common.[117] Electric guitars are used to create feedback, and are played through wah wah and fuzzbox effect pedals.[118] Elaborate studio effects are often used, such as backwards tapes, panning, phasing, long delay loops, and extreme reverb.[119] In the 1960s there was a use of primitive electronic instruments such as early synthesizers and the theremin.[120][121] Later forms of electronic psychedelia also employed repetitive computer-generated beats.[122] Songs allegedly referring to LSD include John Prine's "Illegal Smile" and the Beatles' song "Lucy in the Sky with Diamonds," although the authors of the latter song repeatedly denied this claim.[123][124][125]
In modern times, LSD has had a prominent influence on artists such as Keith Haring, electronic dance music, and the jam band Phish.
Legal status
The United Nations Convention on Psychotropic Substances (adopted in 1971) requires the signing parties to prohibit LSD. Hence, it is illegal in all countries that were parties to the convention, including the United States, Australia, New Zealand, and most of Europe. However, enforcement of those laws varies from country to country. Medical and scientific research with LSD in humans is permitted under the 1971 UN Convention.[126]
Australia
LSD is a Schedule 9 prohibited substance in Australia under the Poisons Standard (February 2017).[127] A Schedule 9 substance is defined as a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[127]
In Western Australia section 9 of the Misuse of Drugs Act 1981 provides for summary trial before a magistrate for possession of less than 0.004g; section 11 provides rebuttable presumptions of intent to sell or supply if the quantity is 0.002g or more, or of possession for the purpose of trafficking if 0.01g.[128]
Canada
In Canada, LSD is a controlled substance under Schedule III of the Controlled Drugs and Substances Act.[39] Every person who seeks to obtain the substance, without disclosing authorization to obtain such substances 30 days before obtaining another prescription from a practitioner, is guilty of an indictable offense and liable to imprisonment for a term not exceeding 3 years. Possession for purpose of trafficking is an indictable offense punishable by imprisonment for 10 years.
United Kingdom
In the United Kingdom, LSD is a Schedule 1 Class 'A' drug. This means it has no recognized legitimate uses and possession of the drug without a license is punishable with 7 years' imprisonment and/or an unlimited fine, and trafficking is punishable with life imprisonment and an unlimited fine (see main article on drug punishments Misuse of Drugs Act 1971).
In 2000, after consultation with members of the Royal College of Psychiatrists' Faculty of Substance Misuse, the UK Police Foundation issued the Runciman Report which recommended "the transfer of LSD from Class A to Class B."[129]
In November 2009, the UK Transform Drug Policy Foundation released in the House of Commons a guidebook to the legal regulation of drugs, After the War on Drugs: Blueprint for Regulation, which details options for regulated distribution and sale of LSD and other psychedelics.[130]
United States
LSD is Schedule I in the United States, according to the Controlled Substances Act of 1970.[131] This means LSD is illegal to manufacture, buy, possess, process, or distribute without a license from the Drug Enforcement Administration (DEA). By classifying LSD as a Schedule I substance, the DEA holds that LSD meets the following three criteria: it is deemed to have a high potential for abuse; it has no legitimate medical use in treatment; and there is a lack of accepted safety for its use under medical supervision. There are no documented deaths from chemical toxicity; most LSD deaths are a result of behavioral toxicity.[132]
There can also be substantial discrepancies between the amount of chemical LSD that one possesses and the amount of possession with which one can be charged in the US. This is because LSD is almost always present in a medium (e.g. blotter or neutral liquid), and in some contexts, the amount that can be considered with respect to sentencing is the total mass of the drug and its medium. This discrepancy was the subject of 1995 United States Supreme Court case, Neal v. United States, which determined that for finding minimum sentence lengths, the total medium weight is used, while for determining the severity of the offense, an estimation of the chemical mass is used.[133]
Lysergic acid and lysergic acid amide, LSD precursors, are both classified in Schedule III of the Controlled Substances Act.[134] Ergotamine tartrate, a precursor to lysergic acid, is regulated under the Chemical Diversion and Trafficking Act.
Mexico
In April 2009, the Mexican Congress approved changes in the General Health Law that decriminalized the possession of illegal drugs for immediate consumption and personal use, allowing a person to possess a moderate amount of LSD. The only restriction is that people in possession of drugs should not be within a 300-meter radius of schools, police departments, or correctional facilities. Marijuana, along with cocaine, opium, heroin, and other drugs were also decriminalized; their possession is not considered a crime as long as the dose does not exceed the limit established in the General Health Law.[135] Many question this, as cocaine is as synthesised as heroin, and both are produced as extracts from plants. The law establishes very low amount thresholds and strictly defines personal dosage. For those arrested with more than the threshold allowed by the law this can result in heavy prison sentences, as they will be assumed to be small traffickers even if there are no other indications that the amount was meant for selling.[136]
Czech Republic
In the Czech Republic, until 31 December 1998 only drug possession "for other person" (i.e. intent to sell) was criminal (apart from production, importation, exportation, offering or mediation, which was and remains criminal) while possession for personal use remained legal.[137]
On 1 January 1999, an amendment of the Criminal Code, which was necessitated in order to align the Czech drug rules with the Single Convention on Narcotic Drugs, became effective, criminalizing possession of "amount larger than small" also for personal use (Art. 187a of the Criminal Code) while possession of small amounts for personal use became a misdemeanor.[137]
The judicial practice came to the conclusion that the "amount larger than small" must be five to ten times larger (depending on drug) than a usual single dose of an average consumer.[138]
Under the Regulation No. 467/2009 Coll, possession of less than 5 doses of LSD was to be considered smaller than large for the purposes of the Criminal Code and was to be treated as a misdemeanor subject to a fine equal to a parking ticket.[139]
Ecuador
According to the 2008 Constitution of Ecuador, in its Article 364, the Ecuadorian state does not see drug consumption as a crime but only as a health concern.[140] Since June 2013 the State drugs regulatory office CONSEP has published a table which establishes maximum quantities carried by persons so as to be considered in legal possession and that person as not a seller of drugs.[140][141][142] The "CONSEP established, at their latest general meeting, that the 0.020 milligrams of LSD shall be considered the maximum consumer amount.[143]
Production

An active dose of LSD is very minute, allowing a large number of doses to be synthesized from a comparatively small amount of raw material. Twenty five kilograms of precursor ergotamine tartrate can produce 5–6 kg of pure crystalline LSD; this corresponds to 100 million doses. Because the masses involved are so small, concealing and transporting illicit LSD is much easier than smuggling cocaine, cannabis, or other illegal drugs.[144]
Manufacturing LSD requires laboratory equipment and experience in the field of organic chemistry. It takes two to three days to produce 30 to 100 grams of pure compound. It is believed that LSD is not usually produced in large quantities, but rather in a series of small batches. This technique minimizes the loss of precursor chemicals in case a step does not work as expected.[144]
Forms

LSD is produced in crystalline form and then mixed with excipients or redissolved for production in ingestible forms. Liquid solution is either distributed in small vials or, more commonly, sprayed onto or soaked into a distribution medium. Historically, LSD solutions were first sold on sugar cubes, but practical considerations forced a change to tablet form. Appearing in 1968 as an orange tablet measuring about 6 mm across, "Orange Sunshine" acid was the first largely available form of LSD after its possession was made illegal. Tim Scully, a prominent chemist, made some of these tablets, but said that most "Sunshine" in the USA came by way of Ronald Stark, who imported approximately thirty-five million doses from Europe.[145]
Over a period of time, tablet dimensions, weight, shape and concentration of LSD evolved from large (4.5–8.1 mm diameter), heavyweight (≥150 mg), round, high concentration (90–350 µg/tab) dosage units to small (2.0–3.5 mm diameter) lightweight (as low as 4.7 µg/tab), variously shaped, lower concentration (12–85 µg/tab, average range 30–40 µg/tab) dosage units. LSD tablet shapes have included cylinders, cones, stars, spacecraft, and heart shapes. The smallest tablets became known as "Microdots."[146]
After tablets came "computer acid" or "blotter paper LSD," typically made by dipping a preprinted sheet of blotting paper into an LSD/water/alcohol solution.[145][146] More than 200 types of LSD tablets have been encountered since 1969 and more than 350 blotter paper designs have been observed since 1975.[146] About the same time as blotter paper LSD came "Windowpane" (AKA "Clearlight"), which contained LSD inside a thin gelatin square a quarter of an inch (6 mm) across.[145] LSD has been sold under a wide variety of often short-lived and regionally restricted street names including Acid, Trips, Uncle Sid, Blotter, Lucy, Alice and doses, as well as names that reflect the designs on the sheets of blotter paper.[37][147] Authorities have encountered the drug in other forms—including powder or crystal, and capsule.[148]
Modern distribution
LSD manufacturers and traffickers in the United States can be categorized into two groups: A few large-scale producers, and an equally limited number of small, clandestine chemists, consisting of independent producers who, operating on a comparatively limited scale, can be found throughout the country.[149] As a group, independent producers are of less concern to the Drug Enforcement Administration than the large-scale groups because their product reaches only local markets.[150]
Many LSD dealers and chemists describe a religious or humanitarian purpose that motivates their illicit activity. Nicholas Schou's book Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World describes one such group, the Brotherhood of Eternal Love. The group was a major American LSD trafficking group in the late 1960s and early 1970s.[151]
In the second half of the 20th century, dealers and chemists loosely associated with the Grateful Dead like Owsley Stanley, Nicholas Sand, Karen Horning, Sarah Maltzer, "Dealer McDope," and Leonard Pickard played an essential role in distributing LSD.[116]
Mimics
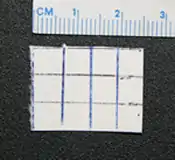

Since 2005, law enforcement in the United States and elsewhere has seized several chemicals and combinations of chemicals in blotter paper which were sold as LSD mimics, including DOB,[152][153] a mixture of DOC and DOI,[154] 25I-NBOMe,[155] and a mixture of DOC and DOB.[156] Street users of LSD are often under the impression that blotter paper which is actively hallucinogenic can only be LSD because that is the only chemical with low enough doses to fit on a small square of blotter paper. While it is true that LSD requires lower doses than most other hallucinogens, blotter paper is capable of absorbing a much larger amount of material. The DEA performed a chromatographic analysis of blotter paper containing 2C-C which showed that the paper contained a much greater concentration of the active chemical than typical LSD doses, although the exact quantity was not determined.[157] Blotter LSD mimics can have relatively small dose squares; a sample of blotter paper containing DOC seized by Concord, California police had dose markings approximately 6 mm apart.[158] Several deaths have been attributed to 25I-NBOMe.[159][160][161][162]
Research
A number of organizations—including the Beckley Foundation, MAPS, Heffter Research Institute and the Albert Hofmann Foundation—exist to fund, encourage and coordinate research into the medicinal and spiritual uses of LSD and related psychedelics.[163] New clinical LSD experiments in humans started in 2009 for the first time in 35 years.[164] As it is illegal in many areas of the world, potential medical uses are difficult to study.[31]
In 2001 the United States Drug Enforcement Administration stated that LSD "produces no aphrodisiac effects, does not increase creativity, has no lasting positive effect in treating alcoholics or criminals, does not produce a 'model psychosis', and does not generate immediate personality change."[165] More recently, experimental uses of LSD have included the treatment of alcoholism[166] and pain and cluster headache relief.[7]
Psychedelic therapy
In the 1950s and 1960s LSD was used in psychiatry to enhance psychotherapy known as psychedelic therapy. Some psychiatrists believed LSD was especially useful at helping patients to "unblock" repressed subconscious material through other psychotherapeutic methods,[167] and also for treating alcoholism.[168][169][170] One study concluded, "The root of the therapeutic value of the LSD experience is its potential for producing self-acceptance and self-surrender,"[171] presumably by forcing the user to face issues and problems in that individual's psyche.
Two recent reviews concluded that conclusions drawn from most of these early trials are unreliable due to serious methodological flaws. These include the absence of adequate control groups, lack of followup, and vague criteria for therapeutic outcome. In many cases studies failed to convincingly demonstrate whether the drug or the therapeutic interaction was responsible for any beneficial effects.[172][173]
In recent years organizations like the Multidisciplinary Association for Psychedelic Studies have renewed clinical research of LSD.[174]
It has been proposed that LSD be studied for use in the therapeutic setting particularly in anxiety.[175]
Other uses
In the 1950s and 1960s, some psychiatrists (e.g. Oscar Janiger) explored the potential effect of LSD on creativity. Experimental studies attempted to measure the effect of LSD on creative activity and aesthetic appreciation.[176][177][178][38]
Since 2008 there has been ongoing research into using LSD to alleviate anxiety for terminally ill cancer patients coping with their impending deaths.[179][180]
A 2012 meta-analysis found evidence that a single dose of LSD in conjunction with various alcoholism treatment programs was associated with a decrease in alcohol abuse, lasting for several months, but no effect was seen at one year. Adverse events included seizure, moderate confusion and agitation, nausea, vomiting, and acting in a bizarre fashion.[33]
LSD has been used as a treatment for cluster headaches with positive results in some small studies.[7]
LSD may have analgesic properties related to pain in terminally ill patients and phantom pain and may be useful for treating inflammatory diseases including rheumatoid arthritis.[181]
Notable individuals
Some notable individuals have commented publicly on their experiences with LSD.[182][183] Some of these comments date from the era when it was legally available in the US and Europe for non-medical uses, and others pertain to psychiatric treatment in the 1950s and 1960s. Still others describe experiences with illegal LSD, obtained for philosophic, artistic, therapeutic, spiritual, or recreational purposes.
- Richard Feynman, a notable physicist at California Institute of Technology, tried LSD during his professorship at Caltech. Feynman largely sidestepped the issue when dictating his anecdotes; he mentions it in passing in the "O Americano, Outra Vez" section.[184][185]
- Jerry Garcia stated in a July 3, 1989 interview for Relix Magazine, in response to the question "Have your feelings about LSD changed over the years?," "They haven't changed much. My feelings about LSD are mixed. It's something that I both fear and that I love at the same time. I never take any psychedelic, have a psychedelic experience, without having that feeling of, "I don't know what's going to happen." In that sense, it's still fundamentally an enigma and a mystery."[186]
- Bill Gates implied in an interview with Playboy that he tried LSD during his youth.[187]
- Aldous Huxley, author of Brave New World, became a user of psychedelics after moving to Hollywood. He was at the forefront of the counterculture's experimentation with psychedelic drugs, which led to his 1954 work The Doors of Perception. Dying from cancer, he asked his wife on 22 November 1963 to inject him with 100 µg of LSD. He died later that day.[188]
- Steve Jobs, co-founder and former CEO of Apple Inc., said, "Taking LSD was a profound experience, one of the most important things in my life."[189]
- In a 2004 interview, Paul McCartney said that The Beatles' songs "Day Tripper" and "Lucy in the Sky with Diamonds" were inspired by LSD trips.[190] Nonetheless, John Lennon consistently stated over the course of many years that the fact that the initials of "Lucy in the Sky with Diamonds" spelled out L-S-D was a coincidence (the title came from a picture drawn by his son Julian) and that the band members did not notice until after the song had been released, and Paul McCartney corroborated that story.[191] John Lennon, George Harrison, and Ringo Starr also experimented with the drug, although McCartney cautioned that "it's easy to overestimate the influence of drugs on the Beatles' music."[192]
- Michel Foucault had an LSD experience with Simeon Wade in the Death Valley and later wrote “it was the greatest experience of his life, and that it profoundly changed his life and his work."[193]
- Kary Mullis is reported to credit LSD with helping him develop DNA amplification technology, for which he received the Nobel Prize in Chemistry in 1993.[194]
- Oliver Sacks, a neurologist famous for writing best-selling case histories about his patients' disorders and unusual experiences, talks about his own experiences with LSD and other perception altering chemicals, in his book, Hallucinations.[195]
See also
Notes
- From the German name Lysergsäure-diethylamid
References
- "Definition of "amide"". Collins English Dictionary. Archived from the original on April 2, 2015. Retrieved January 31, 2015.
- "American Heritage Dictionary Entry: amide". Ahdictionary.com. Archived from the original on April 2, 2015. Retrieved January 31, 2015.
- "amide – definition of amide in English from the Oxford Dictionary". Oxforddictionaries.com. Archived from the original on April 2, 2015. Retrieved January 31, 2015.
- Halpern JH, Suzuki J, Huertas PE, Passie T (June 7, 2014). "Hallucinogen Abuse and Dependence". In Price LH, Stolerman IP (eds.). Encyclopedia of Psychopharmacology A Springer Live Reference. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg. pp. 1–5. doi:10.1007/978-3-642-27772-6_43-2. ISBN 978-3-642-27772-6.
Hallucinogen abuse and dependence are known complications resulting from ... LSD and psilocybin. Users do not experience withdrawal symptoms, but the general criteria for substance abuse and dependence otherwise apply. Dependence is estimated in approximately 2 % of recent-onset users
- Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 375. ISBN 9780071481274.
Several other classes of drugs are categorized as drugs of abuse but rarely produce compulsive use. These include psychedelic agents, such as lysergic acid diethylamide (LSD)
- Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME (June 2015). "Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans". The International Journal of Neuropsychopharmacology. 19 (1): pyv072. doi:10.1093/ijnp/pyv072. PMC 4772267. PMID 26108222.
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
- Neinstein LS (2008). Adolescent Health Care: A Practical Guide. Lippincott Williams & Wilkins. p. 931. ISBN 9780781792561.
- Mucke HA (July 2016). "From Psychiatry to Flower Power and Back Again: The Amazing Story of Lysergic Acid Diethylamide". Assay and Drug Development Technologies. 14 (5): 276–281. doi:10.1089/adt.2016.747. PMID 27392130.
- Kranzler HR, Ciraulo DA (April 2, 2007). Clinical Manual of Addiction Psychopharmacology. American Psychiatric Pub. p. 216. ISBN 9781585626632.
- "What are hallucinogens?". National Institute of Drug Abuse. January 2016. Archived from the original on April 17, 2016. Retrieved April 24, 2016.
- Leptourgos, Pantelis; Fortier-Davy, Martin; Carhart-Harris, Robin; Corlett, Philip R; Dupuis, David; Halberstadt, Adam L; Kometer, Michael; Kozakova, Eva; LarØi, Frank; Noorani, Tehseen N; Preller, Katrin H; Waters, Flavie; Zaytseva, Yuliya; Jardri, Renaud (December 1, 2020). "Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison". Schizophrenia Bulletin. 46 (6): 1399. doi:10.1093/schbul/sbaa117. PMC 7707069. PMID 32944778. Retrieved December 13, 2020.
Thalamocortical connectivity was found altered in psychedelic states. Specifically, LSD was found to selectively increase effective connectivity from the thalamus to certain DMN areas, while other connections are attenuated. Furthermore, increased thalamic connectivity with the right fusiform gyrus and the anterior insula correlated with visual and auditory hallucinations (AH), respectively.
- Orrin Devinsky M.D.; Mark D'Esposito M.D. (October 16, 2003). Neurology of Cognitive and Behavioral Disorders. Oxford University Press. p. 139. ISBN 978-0-19-803148-2.
- "LSD profile (chemistry, effects, other names, synthesis, mode of use, pharmacology, medical use, control status)". EMCDDA. Retrieved July 14, 2018.
- Sloat, Sarah (1/27/2017). "This is Why You Can't Escape an Hours-Long Acid Trip". Inverse. Retrieved 2/3/2020. Check date values in:
|access-date=and|date=(help) - "How LSD Went From Research to Religion | JSTOR Daily". JSTOR Daily. July 19, 2016. Retrieved July 14, 2018.
- Lüscher C, Ungless MA (November 2006). "The mechanistic classification of addictive drugs". PLOS Medicine. 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMC 1635740. PMID 17105338.
- "Commonly Abused Drugs Charts". National Institute on Drug Abuse. July 2, 2018. Retrieved July 14, 2018.
- Halpern JH, Lerner AG, Passie T (2018). A Review of Hallucinogen Persisting Perception Disorder (HPPD) and an Exploratory Study of Subjects Claiming Symptoms of HPPD. Current Topics in Behavioral Neurosciences. 36. pp. 333–360. doi:10.1007/7854_2016_457. ISBN 978-3-662-55878-2. PMID 27822679.
- Abuse, National Institute on Drug. "Hallucinogens". Retrieved July 14, 2018.
- Yockey, R. Andrew; Vidourek, Rebecca A.; King, Keith A. (July 1, 2020). "Trends in LSD use among US adults: 2015–2018". Drug and Alcohol Dependence. 212: 108071. doi:10.1016/j.drugalcdep.2020.108071. ISSN 0376-8716. PMID 32450479.
- "Hallucinogenic effects of LSD discovered". The History Channel. Archived from the original on March 11, 2014.
- Nofil B. "The CIA's Appalling Human Experiments With Mind Control". History Channel. Retrieved July 14, 2018.
- "LSD: cultural revolution and medical advances". Royal Society of Chemistry. Archived from the original on September 30, 2007. Retrieved September 27, 2007.
- Selksy, Andrew; Lieb, David A. (November 4, 2020). "Oregon leads the way in decriminalizing hard drugs". AP NEWS. Retrieved November 18, 2020.
- "DrugFacts: Hallucinogens – LSD, Peyote, Psilocybin, and PCP". National Institute on Drug Abuse. December 2014. Archived from the original on February 16, 2015. Retrieved February 17, 2015.
- "LSD Price: How Much Is Acid Blotter, Sheet, Tab, Pyramid or Vial?". Addiction Resource. Retrieved May 13, 2020.
- Alcohol and Drugs in North America: A Historical Encyclopedia, by David M. Fahey and Jon S. Miller, Editors, ISBN 978-1-59884-478-8, page 375
- San Francisco Chronicle September 20, 1966 Page One
- Grof S, Grof JH (1979). Realms of the Human Unconscious (Observations from LSD Research). London: Souvenir Press (E & A) Ltd. pp. 13–14. ISBN 978-0-285-64882-1. Archived from the original on October 18, 2007. Retrieved November 18, 2007.
- Nutt DJ, King LA, Nichols DE (August 2013). "Effects of Schedule I drug laws on neuroscience research and treatment innovation". Nature Reviews. Neuroscience. 14 (8): 577–85. doi:10.1038/nrn3530. PMID 23756634. S2CID 1956833.
- Campbell D (July 23, 2016). "Scientists study possible health benefits of LSD and ecstasy | Science | The Guardian". The Guardian. Archived from the original on July 23, 2016. Retrieved 2016-07-23.CS1 maint: bot: original URL status unknown (link)
- Krebs TS, Johansen PØ (July 2012). "Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials". Journal of Psychopharmacology. 26 (7): 994–1002. doi:10.1177/0269881112439253. PMID 22406913. S2CID 10677273.
- Dos Santos RG, Osório FL, Crippa JA, Riba J, Zuardi AW, Hallak JE (June 2016). "Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years". Therapeutic Advances in Psychopharmacology. 6 (3): 193–213. doi:10.1177/2045125316638008. PMC 4910400. PMID 27354908.
- NIDA InfoFacts: Hallucinogens – LSD, Peyote, Psilocybin, and PCP Archived November 21, 2009, at the Wayback Machine The National Institute on Drug Abuse (NIDA). Revised 06/09
- Schiff PL (October 2006). "Ergot and its alkaloids". American Journal of Pharmaceutical Education. 70 (5): 98. doi:10.5688/aj700598. PMC 1637017. PMID 17149427.
- Honig, David. Frequently Asked Questions Archived February 12, 2016, at the Wayback Machine via Erowid
- McGlothlin W, Cohen S, McGlothlin MS (November 1967). "Long lasting effects of LSD on normals" (PDF). Archives of General Psychiatry. 17 (5): 521–32. doi:10.1001/archpsyc.1967.01730290009002. PMID 6054248. Archived from the original (PDF) on April 30, 2011.
- Canadian government (1996). "Controlled Drugs and Substances Act". Justice Laws. Canadian Department of Justice. Archived from the original on December 15, 2013. Retrieved December 15, 2013.
- Rogge T (May 21, 2014), Substance use – LSD, MedlinePlus, U.S. National Library of Medicine, archived from the original on July 28, 2016, retrieved July 14, 2016
- CESAR (October 29, 2013), LSD, Center for Substance Abuse Research, University of Maryland, archived from the original on July 15, 2016, retrieved July 14, 2016
- Linton HB, Langs RJ (1962). "Subjective Reactions to Lysergic Acid Diethylamide (LSD-25)" (PDF). Arch. Gen. Psychiatry. 6 (5): 352–68. doi:10.1001/archpsyc.1962.01710230020003. Archived (PDF) from the original on November 18, 2015.
- Katz MM, Waskow IE, Olsson J (February 1968). "Characterizing the psychological state produced by LSD". Journal of Abnormal Psychology. 73 (1): 1–14. CiteSeerX 10.1.1.409.4030. doi:10.1037/h0020114. PMID 5639999.
- Hofmann A. "5. From Remedy to Inebriant". LSD: My Problem Child. Archived from the original on July 17, 2015.
... taste of metal on the palate.
- See, e.g., Gerald Oster's article "Moiré patterns and visual hallucinations Archived October 16, 2015, at the Wayback Machine". Psychedelic Rev. No. 7 (1966): 33–40.
- Nutt D, King LA, Saulsbury W, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/s0140-6736(07)60464-4. PMID 17382831. S2CID 5903121.
- Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–65. CiteSeerX 10.1.1.690.1283. doi:10.1016/s0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M (October 2013), "What can we learn about schizophrenia from studying the human model, drug-induced psychosis?", American Journal of Medical Genetics Part B, 162 (7, Special Issue: Identifying the Origins of Mental Illness: A Festschrift in Honor of Ming T. Tsuang): 661–670, doi:10.1002/ajmg.b.32177, PMID 24132898, S2CID 205326399
- "Is Military Research Hazardous to Veterans Health? Lessons Spanning Half A Century, part F. HALLUCINOGENS". December 8, 1994 John D. Rockefeller IV, West Virginia: 103rd Congress, 2nd Session-S. Prt. 103-97; Staff Report prepared for the committee on veterans' affairs. December 8, 1994. Archived from the original on August 13, 2006. Retrieved December 13, 2018.CS1 maint: location (link)
- Middlefell R (March 1967). "The effects of LSD on body sway suggestibility in a group of hospital patients" (PDF). The British Journal of Psychiatry. 113 (496): 277–80. doi:10.1192/bjp.113.496.277. PMID 6029626. Archived from the original (PDF) on April 30, 2011.
- Sjoberg BM, Hollister LE (November 1965). "The effects of psychotomimetic drugs on primary suggestibility". Psychopharmacologia. 8 (4): 251–62. doi:10.1007/BF00407857. PMID 5885648. S2CID 15249061.
- "LSD, Suggestibility, and Personality Change". Psychology Today. Retrieved May 13, 2020.
- Halpern JH, Pope HG (March 2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug and Alcohol Dependence. 69 (2): 109–19. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.
- Li JH, Lin LF (November 1998). "Genetic toxicology of abused drugs: a brief review". Mutagenesis. 13 (6): 557–65. doi:10.1093/mutage/13.6.557. PMID 9862186.
- Jonas S, Downer J (October 1964). "Gross behavioural changes in monkeys following administration of LSD-25, and development of tolerance to LSD-25". Psychopharmacologia. 6 (4): 303–6. doi:10.1007/BF00413161. PMID 4953438. S2CID 11768927.
- Wolbach AB, Isbell H, Miner EJ (March 1962). "Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions". Psychopharmacologia. 3: 1–14. doi:10.1007/BF00413101. PMID 14007904. S2CID 23803624. Archived from the original on April 19, 2014. Retrieved December 1, 2007.
- Isbell H, Wolbach AB, Wikler A, Miner EJ (1961). "Cross tolerance between LSD and psilocybin". Psychopharmacologia. 2 (3): 147–59. doi:10.1007/BF00407974. PMID 13717955. S2CID 7746880. Archived from the original on March 15, 2016. Retrieved December 1, 2007.
- R.Francis Schlemmer, Carol Nawara,William J. Heinze,John M. Davis, Claire Advokat (1986). "Influence of environmental context on tolerance to LSD-induced behavior in primates". Biol Psychiatry. 21 (3): 314–317. doi:10.1016/0006-3223(86)90053-3. PMID 3947713. S2CID 35508993.CS1 maint: uses authors parameter (link)
- Nichols DE (February 2004). "Hallucinogens". Pharmacology & Therapeutics. 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703. Archived from the original on February 4, 2016. Retrieved January 23, 2006.
- "LSD – National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Archived from the original on March 22, 2018. Retrieved March 21, 2018.
- "LSD (Acid) Fatalities/Deaths". Erowid. Archived from the original on June 1, 2017.
- LSD Toxicity Treatment & Management~treatment at eMedicine
- Marona-Lewicka D, Thisted RA, Nichols DE (July 2005). "Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis". Psychopharmacology. 180 (3): 427–35. doi:10.1007/s00213-005-2183-9. PMID 15723230. S2CID 23565306.
- Nichols D (November 2012). "The End of a Chemistry Era ... Dave Nichols Closes Shop". Archived from the original on September 22, 2013. Retrieved September 24, 2013.
- Aghajanian GK, Bing OH (1964). "Persistence of lysergic acid diethylamide in the plasma of human subjects" (PDF). Clinical Pharmacology and Therapeutics. 5 (5): 611–4. doi:10.1002/cpt196455611. PMID 14209776. S2CID 29438767. Archived from the original (PDF) on March 27, 2009.
- "PDSP database". Archived from the original on May 17, 2013. Retrieved June 28, 2013.
- Nelson DL (February 2004). "5-HT5 receptors". Current Drug Targets. CNS and Neurological Disorders. 3 (1): 53–8. doi:10.2174/1568007043482606. PMID 14965244.
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J (April 2011). "Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists". Neuroscience Letters. 493 (3): 76–9. doi:10.1016/j.neulet.2011.01.046. PMC 3064746. PMID 21276828.
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. (January 2007). "Functional selectivity and classical concepts of quantitative pharmacology". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 1–13. doi:10.1124/jpet.106.104463. PMID 16803859. S2CID 447937.
- BilZ0r. "The Neuropharmacology of Hallucinogens: a technical overview Archived February 4, 2016, at the Wayback Machine". Erowid, v3.1 (August 2005).
- Svenningsson P, Nairn AC, Greengard P (October 2005). "DARPP-32 mediates the actions of multiple drugs of abuse". The AAPS Journal. 7 (2): E353-60. doi:10.1208/aapsj070235. PMC 2750972. PMID 16353915. Archived from the original on October 29, 2012.
- Borroto-Escuela DO, Romero-Fernandez W, Narvaez M, Oflijan J, Agnati LF, Fuxe K (January 2014). "Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes". Biochemical and Biophysical Research Communications. 443 (1): 278–84. doi:10.1016/j.bbrc.2013.11.104. PMID 24309097.
- "PDSP Ki Database". PDSP. Retrieved January 20, 2020.
- Green, J (1977). "Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide". Proc Natl Acad Sci U S A. 74 (12): 5697–5701. Bibcode:1977PNAS...74.5697G. doi:10.1073/pnas.74.12.5697. PMC 431860. PMID 23536.
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (September 5, 2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". Journal of Medicinal Chemistry. 45 (19): 4344–4349. doi:10.1021/jm020153s. PMID 12213075.
- UNC Health Care (January 26, 2017). "This is LSD Attached to a Brain Cell Serotonin Receptor (Update)". Phys.org.
- Cell Press (January 26, 2017). "Structure of LSD and its receptor explains its potency". ScienceDaily.
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, et al. (January 2017). "Crystal Structure of an LSD-Bound Human Serotonin Receptor". Cell. 168 (3): 377–389.e12. doi:10.1016/j.cell.2016.12.033. PMC 5289311. PMID 28129538.
- Alexander and Ann Shulgin. "LSD Archived October 15, 2008, at Wikiwix", in TiHKAL (Berkeley: Transform Press, 1997). ISBN 0-9630096-9-9.
- Hofmann, Albert. LSD—My Problem Child Archived December 15, 2017, at the Wayback Machine (McGraw-Hill, 1980). ISBN 0-07-029325-2.
- Papac DI, Foltz RL (May–June 1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry" (PDF). Journal of Analytical Toxicology. 14 (3): 189–90. doi:10.1093/jat/14.3.189. PMID 2374410. Archived from the original on April 29, 2011.
- Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (March 1995). "Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes". Journal of Medicinal Chemistry. 38 (6): 958–66. doi:10.1021/jm00006a015. PMID 7699712.
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (September 2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". Journal of Medicinal Chemistry. 45 (19): 4344–9. doi:10.1021/jm020153s. PMID 12213075.
- "Erowid Morning Glory Vaults : Extraction of LSA (Method #1)". erowid.org. Archived from the original on September 26, 2014. Retrieved September 25, 2014.
- Kornfeld EC, Fornefeld EJ, Kline GB, Mann MJ, Morrison DE, Jones RG, Woodward RB (1956). "The Total Synthesis of Lysergic Acid". Journal of the American Chemical Society. 78 (13): 3087–3114. doi:10.1021/ja01594a039.
- Inuki S, Oishi S, Fujii N, Ohno H (November 2008). "Total synthesis of (+/-)-lysergic acid, lysergol, and isolysergol by palladium-catalyzed domino cyclization of amino allenes bearing a bromoindolyl group". Organic Letters. 10 (22): 5239–42. doi:10.1021/ol8022648. PMID 18956869.
- Greiner T, Burch NR, Edelberg R (February 1958). "Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum". AMA Archives of Neurology and Psychiatry. 79 (2): 208–10. doi:10.1001/archneurpsyc.1958.02340020088016. PMID 13497365.
- Stoll WA (1947). "Ein neues, in sehr kleinen Mengen wirsames Phantastikum". Arch. Neurol. Schweiz. 60 (4): 483–8. doi:10.1001/archneur.60.4.483. PMID 12707059.
- Erowid & Eduardo Hidalgo, Energy Control (Spain) (2009). "LSD Samples Analysis". Erowid. Archived from the original on February 13, 2010. Retrieved February 8, 2010.
- Henderson LA, Glass WJ (1994). LSD: Still with us after all these years. San Francisco: Jossey-Bass. ISBN 978-0-7879-4379-0.
- Fire & Earth Erowid (2003). "LSD Analysis – Do we know what's in street acid?". Erowid. Archived from the original on January 26, 2010. Retrieved February 8, 2010.
- Li Z, McNally AJ, Wang H, Salamone SJ (October 1998). "Stability study of LSD under various storage conditions". Journal of Analytical Toxicology. 22 (6): 520–5. doi:10.1093/jat/22.6.520. PMID 9788528.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 12th edition, Biomedical Publications, Foster City, CA, 2020, pp. 1197-1199.
- Papac DI, Foltz RL (1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry". Journal of Analytical Toxicology. 14 (3): 189–90. doi:10.1093/jat/14.3.189. PMID 2374410.
- Hofmann 1980, p. 15
- Albert Hofmann; translated from the original German (LSD Ganz Persönlich) by J. Ott. MAPS-Volume 6, Number 69, Summer 1969 Archived 2013-12-06 at the Wayback Machine
- Nichols D (May 24, 2003). "Hypothesis on Albert Hofmann's Famous 1943 "Bicycle Day"". Hofmann Foundation. Archived from the original on September 22, 2007. Retrieved September 27, 2007.
- Hofmann A. "LSD My Problem Child". Archived from the original on January 11, 2010. Retrieved April 19, 2010.
- Hofmann A. "History Of LSD". Archived from the original on September 4, 2007. Retrieved September 27, 2007.
- DEA Public Affairs (November 16, 2001). "LSD: The Drug". Web.petabox.bibalex.org. Archived from the original on April 27, 1999. Retrieved November 27, 2010.
- Nogrady, Thomas; Weaver, Donald F. (2005). Medicinal Chemistry: A Molecular and Biochemical Approach. Oxford University Press. p. 342. ISBN 978-0-19-028296-7.
- "The CIA's Secret Quest For Mind Control: Torture, LSD And A 'Poisoner In Chief'". NPR.org. Retrieved October 6, 2019.
- "Brecher, Edward M; et al. (1972). "How LSD was popularized". Consumer Reports/Drug Library". Druglibrary.org. Archived from the original on May 13, 2012. Retrieved June 20, 2012.
- Anne Applebaum, "Did The Death Of Communism Take Koestler And Other Literary Figures With It? Archived July 14, 2011, at Wikiwix", The Huffington Post, January 26, 2010.
- "Out-Of-Sight! SMiLE Timeline". Archived from the original on February 1, 2010. Retrieved October 30, 2011.
- L. R. Veysey, The Communal Experience: Anarchist and Mystical Communities in Twentieth-Century America (Chicago IL, University of Chicago Press, 1978), ISBN 0-226-85458-2, p. 437.
- United States Congress (October 24, 1968). "Staggers-Dodd Bill, Public Law 90-639" (PDF). Archived (PDF) from the original on May 9, 2010. Retrieved September 8, 2009.
- Gasser P (1994). "Psycholytic Therapy with MDMA and LSD in Switzerland". Archived from the original on October 11, 2009. Retrieved September 8, 2009.
- Feuer, Will (November 4, 2020). "Oregon becomes first state to legalize magic mushrooms as more states ease drug laws in 'psychedelic renaissance'". CNBC.
- J. DeRogatis, Turn On Your Mind: Four Decades of Great Psychedelic Rock (Milwaukie, Michigan: Hal Leonard, 2003), ISBN 0-634-05548-8, pp. 8–9.
- Gilliland J (1969). "Show 41 – The Acid Test: Psychedelics and a sub-culture emerge in San Francisco. [Part 1] : UNT Digital Library" (audio). Pop Chronicles. Digital.library.unt.edu. Archived from the original on June 29, 2011. Retrieved May 6, 2011.
- M. Hicks, Sixties Rock: Garage, Psychedelic, and Other Satisfactions Music in American Life (Chicago, IL: University of Illinois Press, 2000), ISBN 0-252-06915-3, p. 60.
- J. Mann, Turn on and Tune in: Psychedelics, Narcotics and Euphoriants (Royal Society of Chemistry, 2009), ISBN 1-84755-909-3, p. 87.
- Taylor, Michael (March 22, 1996). "OBITUARY -- Ron Thelin". SFGate. Retrieved May 13, 2020.
- Joshua Clark Davis, The Business of Getting High: Head Shops, Countercultural Capitalism, and the Marijuana Legalization Movement. The Sixties: A Journal of Politics, Culture and Society, Summer 2015. Free full text
- Jarnow J (2016). Heads: A Biography of Psychedelic America. Da Capo Press. ISBN 9780306822551.
- Rubin R, Melnick JP (2007). Immigration and American Popular Culture: an Introduction. New York, NY: New York University Press. pp. 162–4. ISBN 978-0-8147-7552-3.
- Prown P, Newquist HP, Eiche JF (1997). Legends of Rock Guitar: the Essential Reference of Rock's Greatest Guitarists. London: Hal Leonard Corporation, 1997. p. 48. ISBN 0-7935-4042-9.
- Borthwick S, Moy R (2004). Popular Music Genres: an Introduction. Edinburgh: Edinburgh University Press. pp. 52–4. ISBN 0-7486-1745-0.
- DeRogatis J (2003). Turn On Your Mind: Four Decades of Great Psychedelic Rock. Milwaukie: Hal Leonard. p. 230. ISBN 0-634-05548-8.
- Unterberger R, Hicks S, Dempsey J (1999). Music USA: the rough guide. Rough Guides. pp. 391. ISBN 1-85828-421-X.
- St John G (2004). Rave Culture and Religion. Abingdon: Routledge. p. 52. ISBN 0-415-31449-6.
- Sheff D (2000). All We Are Saying: The Last Major Interview with John Lennon and Yoko Ono. New York: St. Martin's Press. ISBN 978-0-312-25464-3.
- Thompson T (June 16, 1967). "The New Far-Out Beatles". Life. Chicago: Time Inc. p. 101. Retrieved December 8, 2016.
- McCartney P (June 19, 1967). "Interview with Paul McCartney". ITV Evening News (Interview). London: Independent Television News. Archived from the original on October 15, 2016. Retrieved May 7, 2017.
- UN Convention on Psychotropic Substances, 1971 Final act of the United Nations Conference Archived April 15, 2012, at the Wayback Machine
- Poisons Standard July 2016 Archived March 2, 2017, at the Wayback Machine
- Misuse of Drugs Act 1981 (2015) Slp.wa.gov.au Archived December 22, 2015, at the Wayback Machine
- Drugs and the law: Report of the inquiry into the Misuse of Drugs Act 1971 Archived January 30, 2016, at the Wayback Machine London: Police Foundation, 2000, Runciman Report
- After the War on Drugs: Blueprint for Regulation Archived October 5, 2013, at the Wayback Machine Transform Drug Policy Foundation 2009
- From "Archived copy". Archived from the original on October 5, 2009. Retrieved February 5, 2016.CS1 maint: archived copy as title (link): LSD is a Schedule I substance under the Controlled Substances Act.
- LSD Toxicity at eMedicine
- Neal v. United States, U.S. 284 (1996)., originating from U.S. v. Neal, 46 F.3d 1405 (7th Cir. 1995)
- "Controlled Substances in Alphabetical Order" (PDF). dea.gov. U.S. Drug Enforcement Administration. Retrieved May 29, 2018.
- Ley de Narcomenudeo Archived November 30, 2010, at the Wayback Machine, El Pensador (in Spanish), 17 October 2009
- Mexico: The Law Against Small-Scale Drug Dealing. A Doubtful Venture Archived April 24, 2016, at the Wayback Machine, Jorge Hernández Tinajero & Carlos Zamudio Angles, Series on Legislative Reform of Drug Policies Nr. 3, November 2009
- Parliament of the Czech Republic (1998), Explanatory Report to Act No. 112/1998 Coll., which amends the Act No. 140/1961 Coll., the Criminal Code, and the Act No. 200/1990 Coll., on misdemeanors (in Czech), Prague "Podle čl. 36 Jednotné úmluvy o omamných látkách ze dne 31. března 1961 (č. 47/1965 Sb.) se signatáři zavazují k trestnímu postihu tam uvedených forem nakládání s drogami včetně jejich držby. Návrh upouští od dosavadní beztrestnosti držby omamných a psychotropních látek a jedů pro svoji potřebu. Dosavadní beztrestnost totiž eliminuje v řadě případů možnost postihu dealerů a distributorů drog."
- Supreme Court of the Czech Republic (February 25, 2012), 6 Tdo 156/2010 [NS 7078/2010]
- Government of the Czech Republic (2009), Regulation No. 467/2009 Coll., which defines for the purposes of the Criminal Code what is to be considered larger than small amount of narcotic and psychoactive substances and poisons (in Czech), Prague
- "La nueva tabla para consumo de drogas es una guía para jueces". Archived from the original on June 22, 2013.
- "Dosis máximas de droga para consumo ya están vigentes" Archived June 24, 2013, at the Wayback Machine at El Comercio.com.
- "Ecuador: Aprueban tenencia de drogas para consumo" Archived June 25, 2013, at the Wayback Machine at El Nuevo Herald
- "Ecuador could regulate the drug industry". Archived from the original on June 24, 2013.
- DEA (2007). "LSD Manufacture – Illegal LSD Production". LSD in the United States. U.S. Department of Justice Drug Enforcement Administration. Archived from the original on August 29, 2007.
- Stafford, Peter (1992). "Chapter 1 – The LSD Family". Psychedelics Encyclopaedia (Third Expanded ed.). Ronin Publishing Inc. p. 62. ISBN 978-0-914171-51-5.
- Laing RR, Beyerstein BL, Siegel JA (2003). "Chapter 2.2 – Forms of the Drug". Hallucinogens: A Forensic Drug Handbook. Academic Press. pp. 39–41. ISBN 978-0-12-433951-4.
- "Street Terms: Drugs and the Drug Trade". Office of National Drug Control Policy. April 5, 2005. Archived from the original on April 18, 2009. Retrieved January 31, 2007.
- DEA (2008). "Photo Library (page 2)". US Drug Enforcement Administration. Archived from the original on June 23, 2008. Retrieved June 27, 2008.
- ^ Maclean, J.R.; Macdonald, D.C.; Ogden, F.; Wilby, E., "LSD-25 and mescaline as therapeutic adjuvants." In: Abramson, H., Ed., The Use of LSD in Psychotherapy and Alcoholism, Bobbs-Merrill: New York, 1967, pp. 407–426; Ditman, K.S.; Bailey, J.J., "Evaluating LSD as a psychotherapeutic agent," pp.74–80; Hoffer, A., "A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid," pp. 353–402.
- ^ LSD: The Drug
- Schou N (2010). Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World. Thomas Dunne Books.
- United States Drug Enforcement Administration (October 2005). "LSD Blotter Acid Mimic Containing 4-Bromo-2,5-dimethoxy-amphetamine (DOB) Seized Near Burns, Oregon" (PDF). Microgram Bulletin. 38 (10). Archived from the original (PDF) on October 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (November 2006). "Page 136 MICROGRAM BULLETIN, VOL. XXXIX, NO. 11, NOVEMBER 2006- INTELLIGENCE ALERT -BLOTTER ACID MIMICS (CONTAINING 4-BROMO-2,5-DIMETHOXY-AMPHETAMINE (DOB)) IN CONCORD, CALIFORNIA" (PDF). Microgram Bulletin. 39 (11). Archived from the original (PDF) on October 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (March 2008). "Unusual "Rice Krispie Treat"-Like Balls Containing Psilocybe Mushroom Parts in Warren County, Missouri" (PDF). Microgram Bulletin. 41 (3). Archived from the original (PDF) on October 17, 2012. Retrieved August 20, 2009.
- Iversen L (May 29, 2013). "Temporary Class Drug Order Report on 5-6APB and NBOMe compounds" (PDF). Advisory Council on the Misuse of Drugs. Gov.Uk. p. 14. Archived (PDF) from the original on September 21, 2013. Retrieved June 16, 2013.
- United States Drug Enforcement Administration (March 2009). ""Spice" – Plant Material(s) Laced With Synthetic Cannabinoids or Cannabinoid Mimicking Compounds". Microgram Bulletin. 42 (3). Archived from the original (PDF) on January 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (November 2005). "Bulk Marijuana in Hazardous Packaging in Chicago, Illinois" (PDF). Microgram Bulletin. 38 (11). Archived from the original (PDF) on October 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (December 2007). "SMALL HEROIN DISKS NEAR GREENSBORO, GEORGIA" (PDF). Microgram Bulletin. 40 (12). Archived from the original (PDF) on October 17, 2012. Retrieved August 20, 2009.
- Erowid. "25I-NBOMe (2C-I-NBOMe) Fatalities / Deaths". Drug Website. Erowid. Archived from the original on March 5, 2016. Retrieved February 28, 2016.
- Hastings D (May 6, 2013). "New drug N-bomb hits the street, terrifying parents, troubling cops". New York Daily News. Archived from the original on May 10, 2013. Retrieved May 7, 2013.
- Feehan C (January 21, 2016). "Powerful N-Bomb drug – responsible for spate of deaths internationally – responsible for hospitalisation of six in Cork". Irish Independent. Retrieved January 22, 2016.
- Iversen L (May 29, 2013). "Temporary Class Drug Order Report on 5-6APB and NBOMe compounds" (PDF). Advisory Council on the Misuse of Drugs. Gov.Uk. Archived (PDF) from the original on September 21, 2013. Retrieved June 16, 2013.
- "The Albert Hofmann Foundation". Hofmann Foundation. Archived from the original on July 19, 2019. Retrieved September 27, 2007.
- "LSD & Psilocybin-Assisted Therapy for Anxiety". Archived from the original on May 11, 2018. Retrieved October 16, 2013.
- DEA Public Affairs (November 16, 2001). "DEA – Publications – LSD in the US – The Drug". Web.petabox.bibalex.org. Archived from the original on April 27, 1999. Retrieved November 27, 2010.
- Bogenschutz MP (March 2013). "Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin". Current Drug Abuse Reviews. 6 (1): 17–29. doi:10.2174/15733998113099990002. PMID 23627783.
- Cohen, S. (1959). The therapeutic potential of LSD-25. A Pharmacologic Approach to the Study of the Mind, p251–258.
- "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Retrieved June 20, 2012.
- "Use of d-Lysergic Acid Diethylamide in the Treatment of Alcoholism". Hofmann.org. November 14, 2003. Archived from the original on February 3, 2012. Retrieved February 22, 2012.
- Frood, Arran (March 9, 2012). "LSD helps to treat alcoholism". Nature News. doi:10.1038/nature.2012.10200.
- Chwelos N, Blewett DB, Smith CM, Hoffer A (September 1959). "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Quarterly Journal of Studies on Alcohol. 20 (3): 577–90. doi:10.15288/qjsa.1959.20.577. PMID 13810249.
- Vollenweider FX, Kometer M (September 2010). "The neurobiology of psychedelic drugs: implications for the treatment of mood disorders". Nature Reviews. Neuroscience. 11 (9): 642–51. doi:10.1038/nrn2884. PMID 20717121. S2CID 16588263.
- Baumeister D, Barnes G, Giaroli G, Tracy D (August 2014). "Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles". Therapeutic Advances in Psychopharmacology. 4 (4): 156–69. doi:10.1177/2045125314527985. PMC 4104707. PMID 25083275.
- "LSD-Assisted Psychotherapy". MAPS. Archived from the original on May 11, 2018. Retrieved October 3, 2016.
- Liechti ME (2017). "Modern Clinical Research on LSD". Neuropsychopharmacology. 42 (11): 2114–2127. doi:10.1038/npp.2017.86. PMC 5603820. PMID 28447622.CS1 maint: uses authors parameter (link)
- Sessa B (November 2008). "Is it time to revisit the role of psychedelic drugs in enhancing human creativity?". Journal of Psychopharmacology. 22 (8): 821–7. doi:10.1177/0269881108091597. PMID 18562421. S2CID 1908638.
- Janiger O, Dobkin de Rios M (1989). "LSD and creativity". Journal of Psychoactive Drugs. 21 (1): 129–34. doi:10.1080/02791072.1989.10472150. PMID 2723891. Archived from the original on October 3, 2009.
- Stafford PG, Golightly BH (1967). LSD, the problem-solving psychedelic. ASIN B0006BPSA0. Archived from the original on April 17, 2012.
- "Psychiater Gasser bricht sein Schweigen". Basler Zeitung. July 28, 2009. Archived from the original on October 6, 2011. Retrieved June 19, 2011.
- LSD-Assisted Psychotherapy "LSD-Assisted Psychotherapy". Archived from the original on May 11, 2018. Retrieved February 5, 2017.
- Whelan A, Johnson MI (May 2018). "Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: a potential role?" (PDF). Pain Management. 8 (3): 217–229. doi:10.2217/pmt-2017-0068. PMID 29722608.
- "Famous LSD users". The Good Drugs Guide. Archived from the original on October 7, 2008. Retrieved October 20, 2008.
- "People on psychedelics". Archived from the original on April 21, 2013. Retrieved November 1, 2012.
- Feynman RP (1985). Ralph Leighton (ed.). Surely You're Joking, Mr. Feynman!: Adventures of a Curious Character. W. W. Norton. ISBN 978-0-393-01921-6. OCLC 10925248.
- Gleick J (1992). Genius: The Life and Science of Richard Feynman. Pantheon Books. ISBN 978-0-679-40836-9. OCLC 243743850.
- Alderson J (April 20, 2010). "Q&A with Jerry Garcia: Portrait of an Artist as a Tripper". Relix Magazine. Archived from the original on May 21, 2010. Retrieved June 29, 2013.
- "The Bill Gates Interview". Playboy. July 1994. Archived from the original on July 7, 2014.
- Colman D (October 2011). "Aldous Huxley's LSD Death Trip". Archived from the original on November 12, 2011. Retrieved November 1, 2011.
- Bosker B (October 21, 2011). "The Steve Jobs Reading List: The Books And Artists That Made The Man". Huffington Post. The Huffington Post. Archived from the original on October 22, 2011. Retrieved October 23, 2011.
- Sheff 2000, p. 182.
- "Lucy in the Sky with Diamonds LSD". snopes.com. Retrieved June 20, 2012.
- Matus V (June 2004). "The Truth Behind "LSD"". The Weekly Standard.
- September 15th. "When Michel Foucault Tripped on Acid in Death Valley and Called It "The Greatest Experience of My Life" (1975)". Open Culture. Retrieved April 27, 2019.
- Harrison A (January 16, 2006). "LSD: The Geek's Wonder Drug?". Wired. Archived from the original on May 5, 2008. Retrieved March 11, 2008.
Like Herbert, many scientists and engineers also report heightened states of creativity while using LSD. During a press conference on Friday, Hofmann revealed that he was told by Nobel-prize-winning chemist Kary Mullis that LSD had helped him develop the polymerase chain reaction that helps amplify specific DNA sequences.
- Sacks O (2012). Hallucinations. Vintage Books. p. 106. ISBN 978-0-307-94743-7.
On the West Coast in the early 1960s LSD and morning glory seeds were readily available, so I sampled those, too.
Further reading
- Bebergal P (June 2, 2008). "Will Harvard drop acid again? Psychedelic research returns to Crimsonland". The Phoenix. Boston. Archived from the original on September 20, 2008.
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
External links
| Wikiquote has quotations related to: LSD |
| Wikimedia Commons has media related to LSD. |
- Drug Profiles: LSD European Monitoring Centre for Drugs and Drug Addiction
- InfoFacts – Hallucinogens Archived November 21, 2009, at the Wayback Machine NIDA
- LSD-25 at Erowid
- LSD entry in TiHKAL • info
- U.S. National Library of Medicine: Drug Information Portal – Lysergic acid diethylamide
Documentaries
- Hofmann's Potion a documentary on the origins of LSD, 2002
- Power & Control LSD in The Sixties on YouTube, documentary film directed by Aron Ranen, 2006
- Inside LSD National Geographic Channel, 2009
.svg.png.webp)