Lichen
A lichen (/ˈlaɪkən/ LY-ken or, sometimes in the UK, /ˈlɪtʃən/, LICH-en) is a composite organism that arises from algae or cyanobacteria living among filaments of multiple fungi species[1] in a mutualistic relationship.[2][3][4] Lichens have properties different from those of their component organisms. Lichens come in many colors, sizes, and forms and are sometimes plant-like, but lichens are not plants. Lichens may have tiny, leafless branches (fruticose), flat leaf-like structures (foliose), flakes that lie on the surface like peeling paint (crustose),[5] a powder-like appearance (leprose), or other growth forms.[6]


.jpg.webp)




.jpg.webp)
A macrolichen is a lichen that is either bush-like or leafy; all other lichens are termed microlichens.[2] Here, "macro" and "micro" do not refer to size, but to the growth form.[2] Common names for lichens may contain the word moss (e.g., "reindeer moss", "Iceland moss"), and lichens may superficially look like and grow with mosses, but lichens are not related to mosses or any plant.[4]:3 Lichens do not have roots that absorb water and nutrients as plants do,[7]:2 but like plants, they produce their own nutrition by photosynthesis.[8] When they grow on plants, they do not live as parasites, but instead use the plant's surface as a substrate.
Lichens occur from sea level to high alpine elevations, in many environmental conditions, and can grow on almost any surface.[8] Lichens are abundant growing on bark, leaves, mosses, on other lichens,[7] and hanging from branches "living on thin air" (epiphytes) in rain forests and in temperate woodland. They grow on rock, walls, gravestones, roofs, exposed soil surfaces, rubber, bones, and in the soil as part of biological soil crusts. Different kinds of lichens have adapted to survive in some of the most extreme environments on Earth: arctic tundra, hot dry deserts, rocky coasts, and toxic slag heaps. They can even live inside solid rock, growing between the grains.
It is estimated that 6–8% of Earth's land surface is covered by lichens.[9] There are about 20,000 known species of lichens.[10] Some lichens have lost the ability to reproduce sexually, yet continue to speciate.[7][11] Lichens can be seen as being relatively self-contained miniature ecosystems, where the fungi, algae, or cyanobacteria have the potential to engage with other microorganisms in a functioning system that may evolve as an even more complex composite organism.[12][13][14][15]
Lichens may be long-lived, with some considered to be among the oldest living things.[4][16] They are among the first living things to grow on fresh rock exposed after an event such as a landslide. The long life-span and slow and regular growth rate of some lichens can be used to date events (lichenometry).
Pronunciation and etymology
In American English, "lichen" is pronounced the same as the verb "liken" (/ˈlaɪkən/). In British English, both this pronunciation and one rhyming with "kitchen" /ˈlɪtʃən/) are used.[17][18][19]
English lichen derives from Greek λειχήν leichēn ("tree moss, lichen, lichen-like eruption on skin") via Latin lichen.[20][21][22] The Greek noun, which literally means "licker", derives from the verb λείχειν leichein, "to lick".[23][24]
Like the word moss, the word lichen is also used as an uncountable noun, as in "Lichen grows on rocks".
Growth forms
Lichens grow in a wide range of shapes and forms (morphologies). The shape of a lichen is usually determined by the organization of the fungal filaments.[25] The nonreproductive tissues, or vegetative body parts, are called the thallus. Lichens are grouped by thallus type, since the thallus is usually the most visually prominent part of the lichen. Thallus growth forms typically correspond to a few basic internal structure types. Common names for lichens often come from a growth form or color that is typical of a lichen genus.
Common groupings of lichen thallus growth forms are:
- fruticose[26][27][28] – growing like a tuft or multiple-branched leafless mini-shrub, upright or hanging down, 3-dimensional branches with nearly round cross section (terete) or flattened
- foliose[26][27] – growing in 2-dimensional, flat, leaf-like lobes
- crustose[5][26][27] – crust-like, adhering tightly to a surface (substrate) like a thick coat of paint
- squamulose[28] – formed of small leaf-like scales crustose below but free at the tips
- leprose[29] – powdery
- gelatinous – jelly-like
- filamentous – stringy or like matted hair
- byssoid – wispy, like teased wool
- structureless
There are variations in growth types in a single lichen species, grey areas between the growth type descriptions, and overlapping between growth types, so some authors might describe lichens using different growth type descriptions.
When a crustose lichen gets old, the center may start to crack up like old-dried paint, old-broken asphalt paving, or like the polygonal "islands" of cracked-up mud in a dried lakebed. This is called being rimose or areolate, and the "island" pieces separated by the cracks are called areolas.[26] The areolas appear separated, but are (or were) connected by an underlying "prothallus" or "hypothallus".[29] When a crustose lichen grows from a center and appears to radiate out, it is called crustose placodioid. When the edges of the areolas lift up from the substrate, it is called squamulose.[30]:159[28]
These growth form groups are not precisely defined. Foliose lichens may sometimes branch and appear to be fruticose. Fruticose lichens may have flattened branching parts and appear leafy. Squamulose lichens may appear where the edges lift up. Gelatinous lichens may appear leafy when dry.[30]:159 Means of telling them apart in these cases are in the sections below.
Structures involved in reproduction often appear as discs, bumps, or squiggly lines on the surface of the thallus.[7]:4 The thallus is not always the part of the lichen that is most visually noticeable. Some lichens can grow inside solid rock between the grains (endolithic lichens), with only the sexual fruiting part visible growing outside the rock.[26] These may be dramatic in color or appearance.[26] Forms of these sexual parts are not in the above growth form categories.[26] The most visually noticeable reproductive parts are often circular, raised, plate-like or disc-like outgrowths, with crinkly edges, and are described in sections below.
Color

Lichens come in many colors.[7]:4 Coloration is usually determined by the photosynthetic component.[25] Special pigments, such as yellow usnic acid, give lichens a variety of colors, including reds, oranges, yellows, and browns, especially in exposed, dry habitats.[31] In the absence of special pigments, lichens are usually bright green to olive gray when wet, gray or grayish-green to brown when dry.[31] This is because moisture causes the surface skin (cortex) to become more transparent, exposing the green photobiont layer.[31] Different colored lichens covering large areas of exposed rock surfaces, or lichens covering or hanging from bark can be a spectacular display when the patches of diverse colors "come to life" or "glow" in brilliant displays following rain.
Different colored lichens may inhabit different adjacent sections of a rock face, depending on the angle of exposure to light.[31] Colonies of lichens may be spectacular in appearance, dominating much of the surface of the visual landscape in forests and natural places, such as the vertical "paint" covering the vast rock faces of Yosemite National Park.[32]
Color is used in identification.[33]:4 The color of a lichen changes depending on whether the lichen is wet or dry.[33] Color descriptions used for identification are based on the color that shows when the lichen is dry.[33] Dry lichens with a cyanobacterium as the photosynthetic partner tend to be dark grey, brown, or black.[33]
The underside of the leaf-like lobes of foliose lichens is a different color from the top side (dorsiventral), often brown or black, sometimes white. A fruticose lichen may have flattened "branches", appearing similar to a foliose lichen, but the underside of a leaf-like structure on a fruticose lichen is the same color as the top side. The leaf-like lobes of a foliose lichen may branch, giving the appearance of a fruticose lichen, but the underside will be a different color from the top side.[29]
The sheen on some jelly-like gelatinous lichens is created by mucilaginous secretions.[25]
Internal structure and growth forms
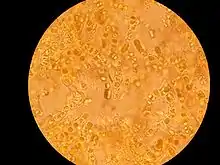
A lichen consists of a simple photosynthesizing organism, usually a green alga or cyanobacterium, surrounded by filaments of a fungus. Generally, most of a lichen's bulk is made of interwoven fungal filaments,[34] although in filamentous and gelatinous lichens[25] this is not the case. The fungus is called a mycobiont. The photosynthesizing organism is called a photobiont. Algal photobionts are called phycobionts.[35] Cyanobacteria photobionts are called cyanobionts.[35]
The part of a lichen that is not involved in reproduction, the "body" or "vegetative tissue" of a lichen, is called the thallus. The thallus form is very different from any form where the fungus or alga are growing separately. The thallus is made up of filaments of the fungus called hyphae. The filaments grow by branching then rejoining to create a mesh, which is called being "anastomose". The mesh of fungal filaments may be dense or loose.
Generally, the fungal mesh surrounds the algal or cyanobacterial cells, often enclosing them within complex fungal tissues that are unique to lichen associations. The thallus may or may not have a protective "skin" of densely packed fungal filaments, often containing a second fungal species,[1] which is called a cortex. Fruticose lichens have one cortex layer wrapping around the "branches". Foliose lichens have an upper cortex on the top side of the "leaf", and a separate lower cortex on the bottom side. Crustose and squamulose lichens have only an upper cortex, with the "inside" of the lichen in direct contact with the surface they grow on (the substrate). Even if the edges peel up from the substrate and appear flat and leaf-like, they lack a lower cortex, unlike foliose lichens. Filamentous, byssoid, leprose,[29] gelatinous, and other lichens do not have a cortex, which is called being ecorticate.[36]
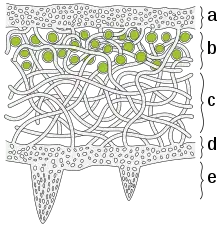
a) The cortex is the outer layer of tightly woven fungus filaments (hyphae)
b) This photobiont layer has photosynthesizing green algae
c) Loosely packed hyphae in the medulla
d) A tightly woven lower cortex
e) Anchoring hyphae called rhizines where the fungus attaches to the substrate
Fruticose, foliose, crustose, and squamulose lichens generally have up to three different types of tissue, differentiated by having different densities of fungal filaments.[34] The top layer, where the lichen contacts the environment, is called a cortex.[34] The cortex is made of densely tightly woven, packed, and glued together (agglutinated) fungal filaments.[34] The dense packing makes the cortex act like a protective "skin", keeping other organisms out, and reducing the intensity of sunlight on the layers below.[34] The cortex layer can be up to several hundred micrometers (μm) in thickness (less than a millimeter).[37] The cortex may be further topped by an epicortex of secretions, not cells, 0.6–1 μm thick in some lichens.[37] This secretion layer may or may not have pores.[37]
Below the cortex layer is a layer called the photobiontic layer or symbiont layer.[27][34] The symbiont layer has less densely packed fungal filaments, with the photosynthetic partner embedded in them.[34] The less dense packing allows air circulation during photosynthesis, similar to the anatomy of a leaf.[34] Each cell or group of cells of the photobiont is usually individually wrapped by hyphae, and in some cases penetrated by a haustorium.[25] In crustose and foliose lichens, algae in the photobiontic layer are diffuse among the fungal filaments, decreasing in gradation into the layer below. In fruticose lichens, the photobiontic layer is sharply distinct from the layer below.[25]
The layer beneath the symbiont layer called is called the medulla. The medulla is less densely packed with fungal filaments than the layers above. In foliose lichens, there is usually, as in Peltigera,[30]:159 another densely packed layer of fungal filaments called the lower cortex.[29][34] Root-like fungal structures called rhizines (usually)[30]:159 grow from the lower cortex to attach or anchor the lichen to the substrate.[2][29] Fruticose lichens have a single cortex wrapping all the way around the "stems" and "branches".[30] The medulla is the lowest layer, and may form a cottony white inner core for the branchlike thallus, or it may be hollow.[30]:159 Crustose and squamulose lichens lack a lower cortex, and the medulla is in direct contact with the substrate that the lichen grows on.
In crustose areolate lichens, the edges of the areolas peel up from the substrate and appear leafy. In squamulose lichens the part of the lichen thallus that is not attached to the substrate may also appear leafy. But these leafy parts lack a lower cortex, which distinguishes crustose and squamulose lichens from foliose lichens.[34] Conversely, foliose lichens may appear flattened against the substrate like a crustose lichen, but most of the leaf-like lobes can be lifted up from the substrate because it is separated from it by a tightly packed lower cortex.[29]
Gelatinous,[30]:159 byssoid, and leprose lichens lack a cortex (are ecorticate), and generally have only undifferentiated tissue, similar to only having a symbiont layer.
In lichens that include both green algal and cyanobacterial symbionts, the cyanobacteria may be held on the upper or lower surface in small pustules called cephalodia.
Pruinia is a whitish coating on top of an upper surface.[38] An epinecral layer is "a layer of horny dead fungal hyphae with indistinct lumina in or near the cortex above the algal layer".[38]
In August 2016, it was reported that macrolichens have more than one species of fungus in their tissues.[1]
Physiology
Symbiotic relation
Lichens are fungi that have discovered agriculture
— Trevor Goward[39]
A lichen is a composite organism that emerges from algae or cyanobacteria living among the filaments (hyphae) of the fungi in a mutually beneficial symbiotic relationship. The fungi benefit from the carbohydrates produced by the algae or cyanobacteria via photosynthesis. The algae or cyanobacteria benefit by being protected from the environment by the filaments of the fungi, which also gather moisture and nutrients from the environment, and (usually) provide an anchor to it. Although some photosynthetic partners in a lichen can survive outside the lichen, the lichen symbiotic association extends the ecological range of both partners, whereby most descriptions of lichen associations describe them as symbiotic. Both partners gain water and mineral nutrients mainly from the atmosphere, through rain and dust. The fungal partner protects the alga by retaining water, serving as a larger capture area for mineral nutrients and, in some cases, provides minerals obtained from the substrate. If a cyanobacterium is present, as a primary partner or another symbiont in addition to a green alga as in certain tripartite lichens, they can fix atmospheric nitrogen, complementing the activities of the green alga.
In three different lineages the fungal partner has independently lost the mitochondrial gene atp9, which has key functions in mitochondrial energy production. The loss makes the fungi completely dependent on their symbionts.[40]
The algal or cyanobacterial cells are photosynthetic and, as in plants, they reduce atmospheric carbon dioxide into organic carbon sugars to feed both symbionts. Phycobionts (algae) produce sugar alcohols (ribitol, sorbitol, and erythritol), which are absorbed by the mycobiont (fungus).[35] Cyanobionts produce glucose.[35] Lichenized fungal cells can make the photobiont "leak" out the products of photosynthesis, where they can then be absorbed by the fungus.[7]:5
It appears many, probably the majority, of lichen also live in a symbiotic relationship with an order of basidiomycete yeasts called Cyphobasidiales. The absence of this third partner could explain the difficulties of growing lichen in the laboratory. The yeast cells are responsible for the formation of the characteristic cortex of the lichen thallus, and could also be important for its shape.[41]
The lichen combination of alga or cyanobacterium with a fungus has a very different form (morphology), physiology, and biochemistry than the component fungus, alga, or cyanobacterium growing by itself, naturally or in culture. The body (thallus) of most lichens is different from those of either the fungus or alga growing separately. When grown in the laboratory in the absence of its photobiont, a lichen fungus develops as a structureless, undifferentiated mass of fungal filaments (hyphae). If combined with its photobiont under appropriate conditions, its characteristic form associated with the photobiont emerges, in the process called morphogenesis.[4] In a few remarkable cases, a single lichen fungus can develop into two very different lichen forms when associating with either a green algal or a cyanobacterial symbiont. Quite naturally, these alternative forms were at first considered to be different species, until they were found growing in a conjoined manner.
Evidence that lichens are examples of successful symbiosis is the fact that lichens can be found in almost every habitat and geographic area on the planet.[12] Two species in two genera of green algae are found in over 35% of all lichens, but can only rarely be found living on their own outside of a lichen.[42]
In a case where one fungal partner simultaneously had two green algae partners that outperform each other in different climates, this might indicate having more than one photosynthetic partner at the same time might enable the lichen to exist in a wider range of habitats and geographic locations.[12]
At least one form of lichen, the North American beard-like lichens, are constituted of not two but three symbiotic partners: an ascomycetous fungus, a photosynthetic alga, and, unexpectedly, a basidiomycetous yeast.[43]
Phycobionts can have a net output of sugars with only water vapor.[35] The thallus must be saturated with liquid water for cyanobionts to photosynthesize.[35]
Algae produce sugars that are absorbed by the fungus by diffusion into special fungal hyphae called appressoria or haustoria in contact with the wall of the algal cells.[44] The appressoria or haustoria may produce a substance that increases permeability of the algal cell walls, and may penetrate the walls.[44] The algae may contribute up to 80% of their sugar production to the fungus.[44]
Ecology
Lichen associations may be examples of mutualism or commensalism, but the lichen relationship can be considered parasitic[45] under circumstances where the photosynthetic partner can exist in nature independently of the fungal partner, but not vice versa. Photobiont cells are routinely destroyed in the course of nutrient exchange. The association continues because reproduction of the photobiont cells matches the rate at which they are destroyed.[45] The fungus surrounds the algal cells,[8] often enclosing them within complex fungal tissues unique to lichen associations. In many species the fungus penetrates the algal cell wall,[8] forming penetration pegs (haustoria) similar to those produced by pathogenic fungi that feed on a host.[28][46] Cyanobacteria in laboratory settings can grow faster when they are alone rather than when they are part of a lichen.
Miniature ecosystem and holobiont theory
Symbiosis in lichens is so well-balanced that lichens have been considered to be relatively self-contained miniature ecosystems in and of themselves.[12][13] It is thought that lichens may be even more complex symbiotic systems that include non-photosynthetic bacterial communities performing other functions as partners in a holobiont.[14][15]
Many lichens are very sensitive to environmental disturbances and can be used to cheaply[8] assess air pollution,[47][48][49] ozone depletion, and metal contamination. Lichens have been used in making dyes, perfumes,[50] and in traditional medicines. A few lichen species are eaten by insects[8] or larger animals, such as reindeer.[51] Lichens are widely used as environmental indicators or bio-indicators. When air is very badly polluted with sulphur dioxide, there may be no lichens present; only some green algae can tolerate those conditions. If the air is clean, then shrubby, hairy and leafy lichens become abundant. A few lichen species can tolerate fairly high levels of pollution, and are commonly found in urban areas, on pavements, walls and tree bark. The most sensitive lichens are shrubby and leafy, while the most tolerant lichens are all crusty in appearance. Since industrialisation, many of the shrubby and leafy lichens such as Ramalina, Usnea and Lobaria species have very limited ranges, often being confined to the areas which have the cleanest air.
Lichenicolous fungi
Some fungi can only be found living on lichens as obligate parasites. These are referred to as lichenicolous fungi, and are a different species from the fungus living inside the lichen; thus they are not considered to be part of the lichen.[52]
Reaction to water
Moisture makes the cortex become more transparent.[7]:4 This way, the algae can conduct photosynthesis when moisture is available, and is protected at other times. When the cortex is more transparent, the algae show more clearly and the lichen looks greener.
Metabolites, metabolite structures and bioactivity
Lichens can show intense antioxidant activity.[53][54] Secondary metabolites are often deposited as crystals in the apoplast.[55] Secondary metabolites are thought to play a role in preference for some substrates over others.[55]
Sometimes lichens contain structures made from fungal metabolites, for example crustose lichens sometimes have a polysaccharide layer in the cortex.
Growth rate
Lichens often have a regular but very slow growth rate of less than a millimeter per year.
In crustose lichens, the area along the margin is where the most active growth is taking place.[30]:159 Most crustose lichens grow only 1–2 mm in diameter per year.
Life span
Lichens may be long-lived, with some considered to be among the oldest living organisms.[4][16] Lifespan is difficult to measure because what defines the "same" individual lichen is not precise.[56] Lichens grow by vegetatively breaking off a piece, which may or may not be defined as the "same" lichen, and two lichens can merge, then becoming the "same" lichen.[56] An Arctic species called "map lichen" (Rhizocarpon geographicum) has been dated at 8,600 years, apparently the world's oldest living organism.[57]
Response to environmental stress
Unlike simple dehydration in plants and animals, lichens may experience a complete loss of body water in dry periods.[8] Lichens are capable of surviving extremely low levels of water content (poikilohydric).[58]:5–6 They quickly absorb water when it becomes available again, becoming soft and fleshy.[8] Reconfiguration of membranes following a period of dehydration requires several minutes or more.
In tests, lichen survived and showed remarkable results on the adaptation capacity of photosynthetic activity within the simulation time of 34 days under Martian conditions in the Mars Simulation Laboratory (MSL) maintained by the German Aerospace Center (DLR).[59][60]
The European Space Agency has discovered that lichens can survive unprotected in space. In an experiment led by Leopoldo Sancho from the Complutense University of Madrid, two species of lichen—Rhizocarpon geographicum and Xanthoria elegans—were sealed in a capsule and launched on a Russian Soyuz rocket 31 May 2005. Once in orbit, the capsules were opened and the lichens were directly exposed to the vacuum of space with its widely fluctuating temperatures and cosmic radiation. After 15 days, the lichens were brought back to earth and were found to be unchanged in their ability to photosynthesize.[61][62]
Reproduction and dispersal
Vegetative reproduction
Many lichens reproduce asexually, either by a piece breaking off and growing on its own (vegetative reproduction) or through the dispersal of diaspores containing a few algal cells surrounded by fungal cells.[2] Because of the relative lack of differentiation in the thallus, the line between diaspore formation and vegetative reproduction is often blurred. Fruticose lichens can easily fragment, and new lichens can grow from the fragment (vegetative reproduction). Many lichens break up into fragments when they dry, dispersing themselves by wind action, to resume growth when moisture returns.[63][64] Soredia (singular: "soredium") are small groups of algal cells surrounded by fungal filaments that form in structures called soralia, from which the soredia can be dispersed by wind.[2] Isidia (singular: "isidium") are branched, spiny, elongated, outgrowths from the thallus that break off for mechanical dispersal.[2] Lichen propagules (diaspores) typically contain cells from both partners, although the fungal components of so-called "fringe species" rely instead on algal cells dispersed by the "core species".[65]
Sexual reproduction

Structures involved in reproduction often appear as discs, bumps, or squiggly lines on the surface of the thallus.[7]:4 Though it has been argued that sexual reproduction in photobiont is selected against, there are strong evidence that suggest meiotic activities (sexual reproduction) in Trebouxia.[66][67] Many lichen fungi reproduce sexually like other fungi, producing spores formed by meiosis and fusion of gametes. Following dispersal, such fungal spores must meet with a compatible algal partner before a functional lichen can form.
Some lichen fungi belong to Basidiomycetes (basidiolichens) and produce mushroom-like reproductive structures resembling those of their nonlichenized relatives.
Most lichen fungi belong to Ascomycetes (ascolichens). Among the ascolichens, spores are produced in spore-producing structures called ascomata.[7] The most common types of ascomata are the apothecium (plural: apothecia) and perithecium (plural: perithecia).[7]:14 Apothecia are usually cups or plate-like discs located on the top surface of the lichen thallus. When apothecia are shaped like squiggly line segments instead of like discs, they are called lirellae.[7]:14 Perithecia are shaped like flasks that are immersed in the lichen thallus tissue, which has a small hole for the spores to escape the flask, and appear like black dots on the lichen surface.[7]:14
The three most common spore body types are raised discs called apothecia (singular: apothecium), bottle-like cups with a small hole at the top called perithecia (singular: perithecium), and pycnidia (singular: pycnidium), shaped like perithecia but without asci (an ascus is the structure that contains and releases the sexual spores in fungi of the Ascomycota).[68]
The apothecium has a layer of exposed spore-producing cells called asci (singular: ascus), and is usually a different color from the thallus tissue.[7]:14 When the apothecium has an outer margin, the margin is called the exciple.[7]:14 When the exciple has a color similar to colored thallus tissue the apothecium or lichen is called lecanorine, meaning similar to members of the genus Lecanora.[7]:14 When the exciple is blackened like carbon it is called lecideine meaning similar to members of the genus Lecidea.[7]:14 When the margin is pale or colorless it is called biatorine.[7]:14

A "podetium" (plural: podetia) is a lichenized stalk-like structure of the fruiting body rising from the thallus, associated with some fungi that produce a fungal apothecium.[27] Since it is part of the reproductive tissue, podetia are not considered part of the main body (thallus), but may be visually prominent.[27] The podetium may be branched, and sometimes cup-like. They usually bear the fungal pycnidia or apothecia or both.[27] Many lichens have apothecia that are visible to the naked eye.[2]
Most lichens produce abundant sexual structures.[69] Many species appear to disperse only by sexual spores.[69] For example, the crustose lichens Graphis scripta and Ochrolechia parella produce no symbiotic vegetative propagules. Instead, the lichen-forming fungi of these species reproduce sexually by self-fertilization (i.e. they are homothallic). This breeding system may enable successful reproduction in harsh environments.[69]
Mazaedia (singular: mazaedium) are apothecia shaped like a dressmaker's pin in (pin lichen)s, where the fruiting body is a brown or black mass of loose ascospores enclosed by a cup-shaped exciple, which sits on top of a tiny stalk.[7]:15
Taxonomy and classification
Lichens are classified by the fungal component. Lichen species are given the same scientific name (binomial name) as the fungus species in the lichen. Lichens are being integrated into the classification schemes for fungi. The alga bears its own scientific name, which bears no relationship to that of the lichen or fungus.[70] There are about 13,500–17,000 identified lichen species.[44] Nearly 20% of known fungal species are associated with lichens.[44]
"Lichenized fungus" may refer to the entire lichen, or to just the fungus. This may cause confusion without context. A particular fungus species may form lichens with different algae species, giving rise to what appear to be different lichen species, but which are still classified (as of 2014) as the same lichen species.[71]
Formerly, some lichen taxonomists placed lichens in their own division, the Mycophycophyta, but this practice is no longer accepted because the components belong to separate lineages. Neither the ascolichens nor the basidiolichens form monophyletic lineages in their respective fungal phyla, but they do form several major solely or primarily lichen-forming groups within each phylum.[72] Even more unusual than basidiolichens is the fungus Geosiphon pyriforme, a member of the Glomeromycota that is unique in that it encloses a cyanobacterial symbiont inside its cells. Geosiphon is not usually considered to be a lichen, and its peculiar symbiosis was not recognized for many years. The genus is more closely allied to endomycorrhizal genera. Fungi from Verrucariales also form marine lichens with the brown algae Petroderma maculiforme,[73] and have a symbiotic relationship with seaweed like (rockweed) and Blidingia minima, where the algae are the dominant components. The fungi is thought to help the rockweeds to resist desiccation when exposed to air.[74][75] In addition, lichens can also use yellow-green algae (Heterococcus) as their symbiotic partner.[76]
Lichens independently emerged from fungi associating with algae and cyanobacteria multiple times throughout history.[77]
Fungi
The fungal component of a lichen is called the mycobiont. The mycobiont may be an Ascomycete or Basidiomycete.[10] The associated lichens are called either ascolichens or basidiolichens, respectively. Living as a symbiont in a lichen appears to be a successful way for a fungus to derive essential nutrients, since about 20% of all fungal species have acquired this mode of life.[78]
Thalli produced by a given fungal symbiont with its differing partners may be similar, and the secondary metabolites identical, indicating that the fungus has the dominant role in determining the morphology of the lichen. But the same mycobiont with different photobionts may also produce very different growth forms.[71] Lichens are known in which there is one fungus associated with two or even three algal species.
Although each lichen thallus generally appears homogeneous, some evidence seems to suggest that the fungal component may consist of more than one genetic individual of that species.
Two or more fungal species can interact to form the same lichen.[79]
The following table lists the orders and families of fungi that include lichen-forming species.
Photobionts
The photosynthetic partner in a lichen is called a photobiont. The photobionts in lichens come from a variety of simple prokaryotic and eukaryotic organisms. In the majority of lichens the photobiont is a green alga (Chlorophyta) or a cyanobacterium. In some lichens both types are present. Algal photobionts are called phycobionts, while cyanobacterial photobionts are called cyanobionts.[35] According to one source, about 90% of all known lichens have phycobionts, and about 10% have cyanobionts,[35] while another source states that two thirds of lichens have green algae as phycobiont, and about one third have a cyanobiont.[28] Approximately 100 species of photosynthetic partners from 40[35] genera and five distinct classes (prokaryotic: Cyanophyceae; eukaryotic: Trebouxiophyceae, Phaeophyceae, Chlorophyceae) have been found to associate with the lichen-forming fungi.[80]
Common algal photobionts are from the genera Trebouxia, Trentepohlia, Pseudotrebouxia, or Myrmecia. Trebouxia is the most common genus of green algae in lichens, occurring in about 40% of all lichens. "Trebouxioid" means either a photobiont that is in the genus Trebouxia, or resembles a member of that genus, and is therefore presumably a member of the class Trebouxiophyceae.[27] The second most commonly represented green alga genus is Trentepohlia.[28] Overall, about 100 species of eukaryotes are known to occur as photobionts in lichens. All the algae are probably able to exist independently in nature as well as in the lichen.[79]
A "cyanolichen" is a lichen with a cyanobacterium as its main photosynthetic component (photobiont).[81] Most cyanolichen are also ascolichens, but a few basidiolichen like Dictyonema and Acantholichen have cyanobacteria as their partner.[82]
The most commonly occurring cyanobacterium genus is Nostoc.[79] Other[28] common cyanobacterium photobionts are from Scytonema.[10] Many cyanolichens are small and black, and have limestone as the substrate. Another cyanolichen group, the jelly lichens of the genera Collema or Leptogium are gelatinous and live on moist soils. Another group of large and foliose species including Peltigera, Lobaria, and Degelia are grey-blue, especially when dampened or wet. Many of these characterize the Lobarion communities of higher rainfall areas in western Britain, e.g., in the Celtic rain forest. Strains of cyanobacteria found in various cyanolichens are often closely related to one another.[83] They differ from the most closely related free-living strains.[83]
The lichen association is a close symbiosis. It extends the ecological range of both partners but is not always obligatory for their growth and reproduction in natural environments, since many of the algal symbionts can live independently. A prominent example is the alga Trentepohlia, which forms orange-coloured populations on tree trunks and suitable rock faces. Lichen propagules (diaspores) typically contain cells from both partners, although the fungal components of so-called "fringe species" rely instead on algal cells dispersed by the "core species".[65]
The same cyanobiont species can occur in association with different fungal species as lichen partners.[84] The same phycobiont species can occur in association with different fungal species as lichen partners.[35] More than one phycobiont may be present in a single thallus.[35]
A single lichen may contain several algal genotypes.[85][86] These multiple genotypes may better enable response to adaptation to environmental changes, and enable the lichen to inhabit a wider range of environments.[87]
Controversy over classification method and species names
There are about 20,000 known lichen species.[10] But what is meant by "species" is different from what is meant by biological species in plants, animals, or fungi, where being the same species implies that there is a common ancestral lineage.[10] Because lichens are combinations of members of two or even three different biological kingdoms, these components must have a different ancestral lineage from each other. By convention, lichens are still called "species" anyway, and are classified according to the species of their fungus, not the species of the algae or cyanobacteria. Lichens are given the same scientific name (binomial name) as the fungus in them, which may cause some confusion. The alga bears its own scientific name, which has no relationship to the name of the lichen or fungus.[70]
Depending on context, "lichenized fungus" may refer to the entire lichen, or to the fungus when it is in the lichen, which can be grown in culture in isolation from the algae or cyanobacteria. Some algae and cyanobacteria are found naturally living outside of the lichen. The fungal, algal, or cyanobacterial component of a lichen can be grown by itself in culture. When growing by themselves, the fungus, algae, or cyanobacteria have very different properties than those of the lichen. Lichen properties such as growth form, physiology, and biochemistry, are very different from the combination of the properties of the fungus and the algae or cyanobacteria.
The same fungus growing in combination with different algae or cyanobacteria, can produce lichens that are very different in most properties, meeting non-DNA criteria for being different "species". Historically, these different combinations were classified as different species. When the fungus is identified as being the same using modern DNA methods, these apparently different species get reclassified as the same species under the current (2014) convention for classification by fungal component. This has led to debate about this classification convention. These apparently different "species" have their own independent evolutionary history.[2][71]
There is also debate as to the appropriateness of giving the same binomial name to the fungus, and to the lichen that combines that fungus with an alga or cyanobacterium (synecdoche). This is especially the case when combining the same fungus with different algae or cyanobacteria produces dramatically different lichen organisms, which would be considered different species by any measure other than the DNA of the fungal component. If the whole lichen produced by the same fungus growing in association with different algae or cyanobacteria, were to be classified as different "species", the number of "lichen species" would be greater.
Diversity
The largest number of lichenized fungi occur in the Ascomycota, with about 40% of species forming such an association.[70] Some of these lichenized fungi occur in orders with nonlichenized fungi that live as saprotrophs or plant parasites (for example, the Leotiales, Dothideales, and Pezizales). Other lichen fungi occur in only five orders in which all members are engaged in this habit (Orders Graphidales, Gyalectales, Peltigerales, Pertusariales, and Teloschistales). Overall, about 98% of lichens have an ascomycetous mycobiont.[88] Next to the Ascomycota, the largest number of lichenized fungi occur in the unassigned fungi imperfecti, a catch-all category for fungi whose sexual form of reproduction has never been observed. Comparatively few Basidiomycetes are lichenized, but these include agarics, such as species of Lichenomphalia, clavarioid fungi, such as species of Multiclavula, and corticioid fungi, such as species of Dictyonema.
Identification methods
Lichen identification uses growth form and reactions to chemical tests.
The outcome of the "Pd test" is called "Pd", which is also used as an abbreviation for the chemical used in the test, para-phenylenediamine.[27] If putting a drop on a lichen turns an area bright yellow to orange, this helps identify it as belonging to either the genus Cladonia or Lecanora.[27]
Evolution and paleontology
The fossil record for lichens is poor.[89] The extreme habitats that lichens dominate, such as tundra, mountains, and deserts, are not ordinarily conducive to producing fossils.[89][90] There are fossilized lichens embedded in amber. The fossilized Anzia is found in pieces of amber in northern Europe and dates back approximately 40 million years.[91] Lichen fragments are also found in fossil leaf beds, such as Lobaria from Trinity County in northern California, USA, dating back to the early to middle Miocene.[92]
The oldest fossil lichen in which both symbiotic partners have been recovered is Winfrenatia, an early zygomycetous (Glomeromycotan) lichen symbiosis that may have involved controlled parasitism, is permineralized in the Rhynie Chert of Scotland, dating from early Early Devonian, about 400 million years ago.[93] The slightly older fossil Spongiophyton has also been interpreted as a lichen on morphological[94] and isotopic[95] grounds, although the isotopic basis is decidedly shaky.[96] It has been demonstrated that Silurian-Devonian fossils Nematothallus[97] and Prototaxites[98] were lichenized. Thus lichenized Ascomycota and Basidiomycota were a component of Early Silurian-Devonian terrestrial ecosystems.[99][100] Newer research suggests that lichen evolved after the evolution of land plants.[101]
The ancestral ecological state of both Ascomycota and Basidiomycota was probably saprobism, and independent lichenization events may have occurred multiple times.[102][103] In 1995, Gargas and colleagues proposed that there were at least five independent origins of lichenization; three in the basidiomycetes and at least two in the Ascomycetes.[104] However, Lutzoni et al. (2001) indicate that lichenization probably evolved earlier and was followed by multiple independent losses. Some non-lichen-forming fungi may have secondarily lost the ability to form a lichen association. As a result, lichenization has been viewed as a highly successful nutritional strategy.[105][106]
Lichenized Glomeromycota may extend well back into the Precambrian. Lichen-like fossils consisting of coccoid cells (cyanobacteria?) and thin filaments (mucoromycotinan Glomeromycota?) are permineralized in marine phosphorite of the Doushantuo Formation in southern China. These fossils are thought to be 551 to 635 million years old or Ediacaran.[107] Ediacaran acritarchs also have many similarities with Glomeromycotan vesicles and spores.[108] It has also been claimed that Ediacaran fossils including Dickinsonia,[109] were lichens,[110] although this claim is controversial.[111] Endosymbiotic Glomeromycota comparable with living Geosiphon may extend back into the Proterozoic in the form of 1500 million year old Horodyskia[112] and 2200 million year old Diskagma.[113] Discovery of these fossils suggest that fungi developed symbiotic partnerships with photoautotrophs long before the evolution of vascular plants, though the Ediacaran lichen hypothesisis largely rejected due to an inappropriate definition of lichens based on taphonomy and substrate ecology.[114]
Ecology and interactions with environment
Substrates and habitats

Lichens cover about 7% of the planet's surface and grow on and in a wide range of substrates and habitats, including some of the most extreme conditions on earth.[115] They are abundant growing on bark, leaves, and hanging from branches "living on thin air" (epiphytes) in rain forests and in temperate woodland. They grow on bare rock, walls, gravestones, roofs, and exposed soil surfaces. They can survive in some of the most extreme environments on Earth: arctic tundra, hot dry deserts, rocky coasts, and toxic slag heaps. They can live inside solid rock, growing between the grains, and in the soil as part of a biological soil crust in arid habitats such as deserts. Some lichens do not grow on anything, living out their lives blowing about the environment.[2]
When growing on mineral surfaces, some lichens slowly decompose their substrate by chemically degrading and physically disrupting the minerals, contributing to the process of weathering by which rocks are gradually turned into soil. While this contribution to weathering is usually benign, it can cause problems for artificial stone structures. For example, there is an ongoing lichen growth problem on Mount Rushmore National Memorial that requires the employment of mountain-climbing conservators to clean the monument.
Lichens are not parasites on the plants they grow on, but only use them as a substrate to grow on. The fungi of some lichen species may "take over" the algae of other lichen species.[8][116] Lichens make their own food from their photosynthetic parts and by absorbing minerals from the environment.[8] Lichens growing on leaves may have the appearance of being parasites on the leaves, but they are not. However, some lichens, notably those of the genus Diploschistes are known to parasitise other lichens. Diploschistes muscorum starts its development in the tissue of a host Cladonia species.[46]:30[28]:171
In the arctic tundra, lichens, together with mosses and liverworts, make up the majority of the ground cover, which helps insulate the ground and may provide forage for grazing animals. An example is "Reindeer moss", which is a lichen, not a moss.[8]
A crustose lichen that grows on rock is called a saxicolous lichen.[27][30]:159 Crustose lichens that grow on the rock are epilithic, and those that grow immersed inside rock, growing between the crystals with only their fruiting bodies exposed to the air, are called endolithic lichens.[26][30]:159[81] A crustose lichen that grows on bark is called a corticolous lichen.[30]:159 A lichen that grows on wood from which the bark has been stripped is called a lignicolous lichen.[36] Lichens that grow immersed inside plant tissues are called endophloidic lichens or endophloidal lichens.[26][30]:159 Lichens that use leaves as substrates, whether the leaf is still on the tree or on the ground, are called epiphyllous or foliicolous.[35] A terricolous lichen grows on the soil as a substrate. Many squamulous lichens are terricolous.[30]:159 Umbillicate lichens are foliose lichens that are attached to the substrate at only one point.[26] A vagrant lichen is not attached to a substrate at all, and lives its life being blown around by the wind.
Lichens and soils
In addition to distinct physical mechanisms by which lichens break down raw stone, recent studies indicate lichens attack stone chemically, entering newly chelated minerals into the ecology.
The lichen exudates, which have powerful chelating capacity, the widespread occurrence of mineral neoformation, particularly metal oxalates, together with the characteristics of weathered substrates, all confirm the significance of lichens as chemical weathering agents.[117]
Over time, this activity creates new fertile soil from lifeless stone.
Lichens may be important in contributing nitrogen to soils in some deserts through being eaten, along with their rock substrate, by snails, which then defecate, putting the nitrogen into the soils.[118] Lichens help bind and stabilize soil sand in dunes.[2] In deserts and semi-arid areas, lichens are part of extensive, living biological soil crusts, essential for maintaining the soil structure.[2] Lichens have a long fossil record in soils dating back 2.2 billion years.[113]
Ecological interactions
Lichens are pioneer species, among the first living things to grow on bare rock or areas denuded of life by a disaster.[2] Lichens may have to compete with plants for access to sunlight, but because of their small size and slow growth, they thrive in places where higher plants have difficulty growing. Lichens are often the first to settle in places lacking soil, constituting the sole vegetation in some extreme environments such as those found at high mountain elevations and at high latitudes.[119] Some survive in the tough conditions of deserts, and others on frozen soil of the Arctic regions.[120]
A major ecophysiological advantage of lichens is that they are poikilohydric (poikilo- variable, hydric- relating to water), meaning that though they have little control over the status of their hydration, they can tolerate irregular and extended periods of severe desiccation. Like some mosses, liverworts, ferns, and a few "resurrection plants", upon desiccation, lichens enter a metabolic suspension or stasis (known as cryptobiosis) in which the cells of the lichen symbionts are dehydrated to a degree that halts most biochemical activity. In this cryptobiotic state, lichens can survive wider extremes of temperature, radiation and drought in the harsh environments they often inhabit.

Lichens do not have roots and do not need to tap continuous reservoirs of water like most higher plants, thus they can grow in locations impossible for most plants, such as bare rock, sterile soil or sand, and various artificial structures such as walls, roofs and monuments. Many lichens also grow as epiphytes (epi- on the surface, phyte- plant) on plants, particularly on the trunks and branches of trees. When growing on plants, lichens are not parasites; they do not consume any part of the plant nor poison it. Lichens produce allelopathic chemicals that inhibit the growth of mosses. Some ground-dwelling lichens, such as members of the subgenus Cladina (reindeer lichens), produce allelopathic chemicals that leach into the soil and inhibit the germination of seeds, spruce and other plants.[121] Stability (that is, longevity) of their substrate is a major factor of lichen habitats. Most lichens grow on stable rock surfaces or the bark of old trees, but many others grow on soil and sand. In these latter cases, lichens are often an important part of soil stabilization; indeed, in some desert ecosystems, vascular (higher) plant seeds cannot become established except in places where lichen crusts stabilize the sand and help retain water.
Lichens may be eaten by some animals, such as reindeer, living in arctic regions. The larvae of a number of Lepidoptera species feed exclusively on lichens. These include common footman and marbled beauty. However, lichens are very low in protein and high in carbohydrates, making them unsuitable for some animals. Lichens are also used by the Northern Flying Squirrel for nesting, food, and a water source during winter.
Effects of air pollution

If lichens are exposed to air pollutants at all times, without any deciduous parts, they are unable to avoid the accumulation of pollutants. Also lacking stomata and a cuticle, lichens may absorb aerosols and gases over the entire thallus surface from which they may readily diffuse to the photobiont layer.[122] Because lichens do not possess roots, their primary source of most elements is the air, and therefore elemental levels in lichens often reflect the accumulated composition of ambient air. The processes by which atmospheric deposition occurs include fog and dew, gaseous absorption, and dry deposition.[123] Consequently, many environmental studies with lichens emphasize their feasibility as effective biomonitors of atmospheric quality.[122][124][125][126][127][128]
Not all lichens are equally sensitive to air pollutants, so different lichen species show different levels of sensitivity to specific atmospheric pollutants.[129] The sensitivity of a lichen to air pollution is directly related to the energy needs of the mycobiont, so that the stronger the dependency of the mycobiont on the photobiont, the more sensitive the lichen is to air pollution.[130] Upon exposure to air pollution, the photobiont may use metabolic energy for repair of its cellular structures that would otherwise be used for maintenance of its photosynthetic activity, therefore leaving less metabolic energy available for the mycobiont. The alteration of the balance between the photobiont and mycobiont can lead to the breakdown of the symbiotic association. Therefore, lichen decline may result not only from the accumulation of toxic substances, but also from altered nutrient supplies that favor one symbiont over the other.[122]
This interaction between lichens and air pollution has been used as a means of monitoring air quality since 1859, with more systematic methods developed by William Nylander in 1866.[2]
Human use

Food
Lichens are eaten by many different cultures across the world. Although some lichens are only eaten in times of famine, others are a staple food or even a delicacy. Two obstacles are often encountered when eating lichens: lichen polysaccharides are generally indigestible to humans, and lichens usually contain mildly toxic secondary compounds that should be removed before eating. Very few lichens are poisonous, but those high in vulpinic acid or usnic acid are toxic.[131] Most poisonous lichens are yellow.
In the past, Iceland moss (Cetraria islandica) was an important source of food for humans in northern Europe, and was cooked as a bread, porridge, pudding, soup, or salad. Wila (Bryoria fremontii) was an important food in parts of North America, where it was usually pitcooked. Northern peoples in North America and Siberia traditionally eat the partially digested reindeer lichen (Cladina spp.) after they remove it from the rumen of caribou or reindeer that have been killed. Rock tripe (Umbilicaria spp. and Lasalia spp.) is a lichen that has frequently been used as an emergency food in North America, and one species, Umbilicaria esculenta, is used in a variety of traditional Korean and Japanese foods.
Lichenometry
Lichenometry is a technique used to determine the age of exposed rock surfaces based on the size of lichen thalli. Introduced by Beschel in the 1950s,[132] the technique has found many applications. it is used in archaeology, palaeontology, and geomorphology. It uses the presumed regular but slow rate of lichen growth to determine the age of exposed rock.[32]:9[133] Measuring the diameter (or other size measurement) of the largest lichen of a species on a rock surface indicates the length of time since the rock surface was first exposed. Lichen can be preserved on old rock faces for up to 10,000 years, providing the maximum age limit of the technique, though it is most accurate (within 10% error) when applied to surfaces that have been exposed for less than 1,000 years.[134] Lichenometry is especially useful for dating surfaces less than 500 years old, as radiocarbon dating techniques are less accurate over this period.[135] The lichens most commonly used for lichenometry are those of the genera Rhizocarpon (e.g. the species Rhizocarpon geographicum) and Xanthoria.
Biodegradation
Lichens have been shown to degrade polyester resins, as can be seen in archaeological sites in the Roman city of Baelo Claudia in Spain.[136] Lichens can accumulate several environmental pollutants such as lead, copper, and radionuclides.[137] Some species of lichen such as Parmelia sulcata and Lobaria pulmonaria, and many in the Cladonia genus have been shown to produce serine proteases capable of the degradation of pathogenic forms of prion protein (PrP), which may be useful in treating contaminated environmental reservoirs.[138][139][140]
As dyes
Many lichens produce secondary compounds, including pigments that reduce harmful amounts of sunlight and powerful toxins that reduce herbivory or kill bacteria. These compounds are very useful for lichen identification, and have had economic importance as dyes such as cudbear or primitive antibiotics.
The pH indicator (indicated acidic or basic) in the litmus test is a dye extracted from the lichen Roccella tinctoria by boiling.
In the Highlands of Scotland, traditional dyes for Harris tweed[2] and other traditional cloths were made from lichens, including the orange Xanthoria parietina and the grey foliaceous Parmelia saxatilis common on rocks known as "crottle".
There are reports dating almost 2,000 years old of lichens being used to make purple and red dyes.[141] Of great historical and commercial significance are lichens belonging to the family Roccellaceae, commonly called orchella weed or orchil. Orcein and other lichen dyes have largely been replaced by synthetic versions.
Traditional medicine and research
Historically in traditional medicine of Europe, Lobaria pulmonaria was collected in large quantities as "Lungwort", due to its lung-like appearance (the doctrine of signatures suggesting that herbs can treat body parts that they physically resemble). Similarly, Peltigera leucophlebia was used as a supposed cure for thrush, due to the resemblance of its cephalodia to the appearance of the disease.[28]
Lichens produce metabolites in research for their potential therapeutic or diagnostic value.[142] Some metabolites produced by lichens are structurally and functionally similar to broad-spectrum antibiotics while few are associated respectively to antiseptic similarities.[143] Usnic acid is the most commonly studied metabolite produced by lichens.[143] It is also under research as an bactericidal agent against Escherichia coli and Staphylococcus aureus.[144]
Aesthetic appeal

Colonies of lichens may be spectacular in appearance, dominating the surface of the visual landscape as part of the aesthetic appeal to visitors of Yosemite National Park, Sequoia National Park and the Bay of Fires.[32]:2 Orange and yellow lichens add to the ambience of desert trees, rock faces, tundras, and rocky seashores. Intricate webs of lichens hanging from tree branches add a mysterious aspect to forests. Fruticose lichens are used in model railroading[145] and other modeling hobbies as a material for making miniature trees and shrubs.
In literature
In early Midrashic literature, the Hebrew word "vayilafeth" in Ruth 3:8 is explained as referring to Ruth entwining herself around Boaz like lichen.[146] The tenth century Arab physician, Al-Tamimi, mentions lichens dissolved in vinegar and rose water being used in his day for the treatment of skin diseases and rashes.[147]
The plot of John Wyndham's novel Trouble with Lichen revolves around an anti-aging chemical extracted from a lichen.
History
Although lichens had been recognized as organisms for quite some time, it was not until 1867, when Swiss botanist Simon Schwendener proposed his dual theory of lichens, that lichens are a combination of fungi with algae or cyanobacteria, whereby the true nature of the lichen association began to emerge.[148] Schwendener's hypothesis, which at the time lacked experimental evidence, arose from his extensive analysis of the anatomy and development in lichens, algae, and fungi using a light microscope. Many of the leading lichenologists at the time, such as James Crombie and Nylander, rejected Schwendener's hypothesis because the common consensus was that all living organisms were autonomous.[148]
Other prominent biologists, such as Heinrich Anton de Bary, Albert Bernhard Frank, Melchior Treub and Hermann Hellriegel were not so quick to reject Schwendener's ideas and the concept soon spread into other areas of study, such as microbial, plant, animal and human pathogens.[148][149] When the complex relationships between pathogenic microorganisms and their hosts were finally identified, Schwendener's hypothesis began to gain popularity. Further experimental proof of the dual nature of lichens was obtained when Eugen Thomas published his results in 1939 on the first successful re-synthesis experiment.[148]
In the 2010s, a new facet of the fungi-algae partnership was discovered. Toby Spribille and colleagues found that many types of lichen that were long thought to be ascomycete-algae pairs were actually ascomycete-basidiomycete-algae trios.[1][150]
Gallery
 Lobaria pulmonaria, tree lungwort, lung lichen, lung moss; Upper Bavaria, Germany
Lobaria pulmonaria, tree lungwort, lung lichen, lung moss; Upper Bavaria, Germany Cladonia macilenta var. bacillaris 'Lipstick Cladonia'
Cladonia macilenta var. bacillaris 'Lipstick Cladonia' Usnea australis, a fruticose form, growing on a tree branch
Usnea australis, a fruticose form, growing on a tree branch Hypogymnia cf. tubulosa with Bryoria sp. and Tuckermannopsis sp. in the Canadian Rockies
Hypogymnia cf. tubulosa with Bryoria sp. and Tuckermannopsis sp. in the Canadian Rockies Letharia sp. with Bryoria sp. on pine branches near Blackpine Lake, Washington State
Letharia sp. with Bryoria sp. on pine branches near Blackpine Lake, Washington State Lobaria oregana, commonly called 'Lettuce lichen', in the Hoh Rainforest, Washington State
Lobaria oregana, commonly called 'Lettuce lichen', in the Hoh Rainforest, Washington State
 A lichen growing on a rock in a Brazilian cloud forest
A lichen growing on a rock in a Brazilian cloud forest Xanthoparmelia cf. lavicola, a foliose lichen, on basalt.
Xanthoparmelia cf. lavicola, a foliose lichen, on basalt. Map lichen (Rhizocarpon geographicum) on rock
Map lichen (Rhizocarpon geographicum) on rock Physcia millegrana (a foliose lichen), with an unlichenized polypore fungus (bottom right), on a fallen log.
Physcia millegrana (a foliose lichen), with an unlichenized polypore fungus (bottom right), on a fallen log. Reindeer moss (Cladonia rangiferina)
Reindeer moss (Cladonia rangiferina) Crustose lichens on limestone in Alta Murgia-Southern Italy
Crustose lichens on limestone in Alta Murgia-Southern Italy Cladonia cf. cristatella, a lichen commonly referred to as 'British Soldiers'. Notice the red tips.
Cladonia cf. cristatella, a lichen commonly referred to as 'British Soldiers'. Notice the red tips.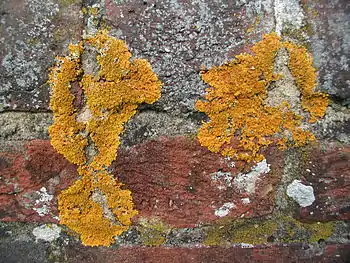 A crusty crustose lichen on a wall
A crusty crustose lichen on a wall Lichen on a lilac bush
Lichen on a lilac bush Foliose lichens on rock growing outward and dying in the center. These lichens are at least several decades old.
Foliose lichens on rock growing outward and dying in the center. These lichens are at least several decades old. Xanthoria sp. lichen on volcanic rock in Craters of the Moon National Monument (Idaho, USA)
Xanthoria sp. lichen on volcanic rock in Craters of the Moon National Monument (Idaho, USA)
.jpg.webp) Microscopic view of lichen growing on a piece of concrete dust.[note 1]
Microscopic view of lichen growing on a piece of concrete dust.[note 1]
See also
Notes
- This was scraped from a dry, concrete-paved section of a drainage ditch. This entire image covers a square that is approximately 1.7 millimeters on a side. The numbered ticks on the scale represent distances of 230 micrometers, or slightly less than 0.25 millimeter.
References
- Spribille, Toby; Tuovinen, Veera; Resl, Philipp; Vanderpool, Dan; Wolinski, Heimo; Aime, M. Catherine; Schneider, Kevin; Stabentheiner, Edith; Toome-Heller, Merje (21 July 2016). "Basidiomycete yeasts in the cortex of ascomycete macrolichens". Science. 353 (6298): 488–92. Bibcode:2016Sci...353..488S. doi:10.1126/science.aaf8287. ISSN 0036-8075. PMC 5793994. PMID 27445309.
- "What is a lichen?". Australian National Botanic Gardens. Archived from the original on 2 July 2014. Retrieved 10 October 2014.
- Introduction to Lichens – An Alliance between Kingdoms Archived 22 August 2014 at the Wayback Machine. University of California Museum of Paleontology.
- Brodo, Irwin M. and Duran Sharnoff, Sylvia (2001) Lichens of North America. ISBN 978-0300082494.
- Galloway, D.J. (13 May 1999). "Lichen Glossary". Australian National Botanic Gardens. Archived from the original on 6 December 2014.
- Margulis, Lynn; Barreno, EVA (2003). "Looking at Lichens". BioScience. 53 (8): 776. doi:10.1641/0006-3568(2003)053[0776:LAL]2.0.CO;2.
- Sharnoff, Stephen (2014) Field Guide to California Lichens, Yale University Press. ISBN 978-0-300-19500-2
- Speer, Brian R; Ben Waggoner (May 1997). "Lichens: Life History & Ecology". University of California Museum of Paleontology. Archived from the original on 2 May 2015. Retrieved 28 April 2015.
- Asplund, Johan; Wardle, David A. (11 October 2016). "How lichens impact on terrestrial community and ecosystem properties". Biological Reviews. 92 (3): 1720–1738. doi:10.1111/brv.12305. hdl:11250/2578209. ISSN 1464-7931. PMID 27730713. S2CID 25453156.
- "Lichens: Systematics, University of California Museum of Paleontology". Archived from the original on 24 February 2015. Retrieved 10 October 2014.
- Lendemer, J. C. (2011). "A taxonomic revision of the North American species of Lepraria s.l. that produce divaricatic acid, with notes on the type species of the genus L. incana". Mycologia. 103 (6): 1216–1229. doi:10.3852/11-032. PMID 21642343. S2CID 34346229.
- Casano, L. M.; Del Campo, E. M.; García-Breijo, F. J.; Reig-Armiñana, J; Gasulla, F; Del Hoyo, A; Guéra, A; Barreno, E (2011). "Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition?". Environmental Microbiology (Submitted manuscript). 13 (3): 806–818. doi:10.1111/j.1462-2920.2010.02386.x. hdl:10251/60269. PMID 21134099.
- Honegger, R. (1991) Fungal evolution: symbiosis and morphogenesis, Symbiosis as a Source of Evolutionary Innovation, Margulis, L., and Fester, R. (eds). Cambridge, MA, USA: The MIT Press, pp. 319–340.
- Grube, M; Cardinale, M; De Castro Jr, J. V.; Müller, H; Berg, G (2009). "Species-specific structural and functional diversity of bacterial communities in lichen symbioses". The ISME Journal. 3 (9): 1105–1115. doi:10.1038/ismej.2009.63. PMID 19554038.
- Barreno, E., Herrera-Campos, M., García-Breijo, F., Gasulla, F., and Reig-Armiñana, J. (2008) "Non photosynthetic bacteria associated to cortical structures on Ramalinaand Usnea thalli from Mexico". Asilomar, Pacific Grove, CA, USA: Abstracts IAL 6- ABLS Joint Meeting.
- Morris J, Purvis W (2007). Lichens (Life). London: The Natural History Museum. p. 19. ISBN 978-0-565-09153-8.
- "Lichen". spectator.co.uk. 17 November 2012. Archived from the original on 23 December 2014. Retrieved 2 November 2014.
- "Lichen". Oxford Living Dictionary. Oxford University Press. Archived from the original on 29 August 2014. Retrieved 10 January 2018.
- The Oxford English Dictionary cites only the "liken" pronunciation: "lichen". Oxford English Dictionary (Online ed.). Oxford University Press. Retrieved 10 January 2018. (Subscription or participating institution membership required.)
- Harper, Douglas. "lichen". Online Etymology Dictionary.
- lichen. Charlton T. Lewis and Charles Short. A Latin Dictionary on Perseus Project.
- λειχήν. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- λείχειν in Liddell and Scott.
- Beekes, Robert S. P. (2010). "s.v. λειχήν, λείχω". Etymological Dictionary of Greek. Leiden Indo-European Etymological Dictionary Series. 1. With the assistance of Lucien van Beek. Leiden, Boston: Brill. pp. 846–47. ISBN 9789004174184.
- "Lichens and Bryophytes, Michigan State University, 10-25-99". Archived from the original on 5 October 2011. Retrieved 10 October 2014.
- Lichen Vocabulary, Lichens of North America Information, Sylvia and Stephen Sharnoff, Archived 20 January 2015 at the Wayback Machine
- "Alan Silverside's Lichen Glossary (p-z), Alan Silverside". Archived from the original on 31 October 2014. Retrieved 10 October 2014.
- Dobson, F.S. (2011). Lichens, an illustrated guide to the British and Irish species. Slough, UK: Richmond Publishing Co. ISBN 9780855463151.
- "Foliose lichens, Lichen Thallus Types, Allan Silverside". Archived from the original on 19 October 2014. Retrieved 10 October 2014.
- Mosses Lichens & Ferns of Northwest North America, Dale H. Vitt, Janet E. Marsh, Robin B. Bovey, Lone Pine Publishing Company, ISBN 0-295-96666-1
- "Lichens, Saguaro-Juniper Corporation". Archived from the original on 10 May 2015. Retrieved 10 October 2014.
- McCune, B.; Grenon, J.; Martin, E.; Mutch, L.S.; Martin, E.P. (March 2007). "Lichens in relation to management issues in the Sierra Nevada national parks". North American Fungi. 2: 1–39. doi:10.2509/pnwf.2007.002.003.
- Michigan Lichens, Julie Jones Medlin, B. Jain Publishers, 1996, ISBN 0877370397, 9780877370390, Archived 24 November 2016 at the Wayback Machine
- Lichens: More on Morphology, University of California Museum of Paleontology, Archived 28 February 2015 at the Wayback Machine
- Lichen Photobionts, University of Nebraska Omaha Archived 6 October 2014 at the Wayback Machine
- "Alan Silverside's Lichen Glossary (g-o), Alan Silverside". Archived from the original on 2 November 2014. Retrieved 10 October 2014.
- Büdel, B.; Scheidegger, C. (1996). Thallus morphology and anatomy. Lichen Biology. pp. 37–64. doi:10.1017/CBO9780511790478.005. ISBN 9780511790478.
- Heiđmarsson, Starri; Heidmarsson, Starri (1996). "Pruina as a Taxonomic Character in the Lichen Genus Dermatocarpon". The Bryologist. 99 (3): 315–320. doi:10.2307/3244302. JSTOR 3244302.
- Sharnoff, Sylvia and Sharnoff, Stephen. "Lichen Biology and the Environment" Archived 17 October 2015 at the Wayback Machine. sharnoffphotos.com
- Pogoda, C. S.; Keepers, K. G.; Lendemer, J. C.; Kane, N. C.; Tripp, E. A. (2018). "Reductions in complexity of mitochondrial genomes in lichen‐forming fungi shed light on genome architecture of obligate symbioses – Wiley Online Library". Molecular Ecology. 27 (5): 1155–1169. doi:10.1111/mec.14519. PMID 29417658.
- Basidiomycete yeasts in the cortex of ascomycete macrolichens – Science
- Skaloud, P; Peksa, O (2010). "Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta)". Molecular Phylogenetics and Evolution. 54 (1): 36–46. doi:10.1016/j.ympev.2009.09.035. PMID 19853051.
- Spribille, Toby; Tuovinen, Veera; Resl, Philipp; Vanderpool, Dan; Wolinski, Heimo; Aime, M. Catherine; Schneider, Kevin; Stabentheiner, Edith; Toome-Heller, Merje; Thor, Göran; Mayrhofer, Helmut (29 July 2016). "Basidiomycete yeasts in the cortex of ascomycete macrolichens". Science. 353 (6298): 488–492. Bibcode:2016Sci...353..488S. doi:10.1126/science.aaf8287. ISSN 0036-8075. PMID 27445309.
- Ramel, Gordon. "What is a Lichen?". Earthlife Web. Archived from the original on 19 January 2015. Retrieved 20 January 2015.
- Ahmadjian V. (1993). The Lichen Symbiosis. New York: John Wiley & Sons. ISBN 978-0-471-57885-7.
- Honegger, R. (1988). "Mycobionts". In Nash III, T.H. (ed.). Lichen Biology. Cambridge: Cambridge University Press (published 1996). ISBN 978-0-521-45368-4.
- Ferry, B. W., Baddeley, M. S. & Hawksworth, D. L. (editors) (1973) Air Pollution and Lichens. Athlone Press, London.
- Rose C. I., Hawksworth D. L. (1981). "Lichen recolonization in London's cleaner air". Nature. 289 (5795): 289–292. Bibcode:1981Natur.289..289R. doi:10.1038/289289a0. S2CID 4320709.
- Hawksworth, D.L. and Rose, F. (1976) Lichens as pollution monitors. Edward Arnold, Institute of Biology Series, No. 66. ISBN 0713125551
- "Oak Moss Absolute Oil, Evernia prunastri, Perfume Fixative". Archived from the original on 25 December 2014. Retrieved 19 September 2014.
- Skogland, Terje (1984). "Wild reindeer foraging-niche organization". Ecography. 7 (4): 345. doi:10.1111/j.1600-0587.1984.tb01138.x.
- Lawrey, James D.; Diederich, Paul (2003). "Lichenicolous Fungi: Interactions, Evolution, and Biodiversity" (PDF). The Bryologist. 106: 80. doi:10.1639/0007-2745(2003)106[0080:LFIEAB]2.0.CO;2. Archived (PDF) from the original on 3 January 2011. Retrieved 2 May 2011.
- Hagiwara K, Wright PR, et al. (March 2015). "Comparative analysis of the antioxidant properties of Icelandic and Hawaiian lichens". Environmental Microbiology. 18 (8): 2319–2325. doi:10.1111/1462-2920.12850. PMID 25808912. S2CID 13768322.
- Odabasoglu F, Aslan A, Cakir A, et al. (March 2005). "Antioxidant activity, reducing power and total phenolic content of some lichen species". Fitoterapia. 76 (2): 216–219. doi:10.1016/j.fitote.2004.05.012. PMID 15752633.
- Hauck, Markus; Jürgens, Sascha-René; Leuschner, Christoph (2010). "Norstictic acid: Correlations between its physico-chemical characteristics and ecological preferences of lichens producing this depsidone". Environmental and Experimental Botany. 68 (3): 309. doi:10.1016/j.envexpbot.2010.01.003.
- "The Earth Life Web, Growth and Development in Lichens". earthlife.net. Archived from the original on 28 May 2015. Retrieved 12 October 2014.
- "Lichens". National Park Service, US Department of the Interior, Government of the United States. 22 May 2016. Archived from the original on 5 April 2018. Retrieved 4 April 2018.
- Nash III, Thomas H. (2008). "Introduction". In Nash III, T.H. (ed.). Lichen Biology (2nd ed.). Cambridge: Cambridge University Press. pp. 1–8. doi:10.1017/CBO9780511790478.002. ISBN 978-0-521-69216-8.
- Baldwin, Emily (26 April 2012). "Lichen survives harsh Mars environment". Skymania News. Archived from the original on 28 May 2012. Retrieved 27 April 2012.
- Sheldrake, Merlin (2020). Entangled Life: How Fungi Make Our Worlds, Change Our Minds and Shape Our Futures. Bodley Head. p. 94. ISBN 978-1847925206.
- "ESA — Human Spaceflight and Exploration – Lichen survives in space". Archived from the original on 26 February 2010. Retrieved 16 February 2010.
- Sancho, L. G.; De La Torre, R.; Horneck, G.; Ascaso, C.; De Los Rios, A.; Pintado, A.; Wierzchos, J.; Schuster, M. (2007). "Lichens survive in space: results from the 2005 LICHENS experiment". Astrobiology. 7 (3): 443–454. Bibcode:2007AsBio...7..443S. doi:10.1089/ast.2006.0046. PMID 17630840. S2CID 4121180.
- Eichorn, Susan E., Evert, Ray F., and Raven, Peter H. (2005). Biology of Plants. New York: W. H. Freeman and Company. p. 1. ISBN 0716710072.
- Cook, Rebecca; McFarland, Kenneth (1995). General Botany 111 Laboratory Manual. Knoxville, TN: University of Tennessee. p. 104.
- A. N. Rai; B. Bergman; Ulla Rasmussen (31 July 2002). Cyanobacteria in Symbiosis. Springer. p. 59. ISBN 978-1-4020-0777-4. Archived from the original on 31 December 2013. Retrieved 2 June 2013.
- Law, R.; Lewis, D. H. (November 1983). "Biotic environments and the maintenance of sex-some evidence from mutualistic symbioses". Biological Journal of the Linnean Society. 20 (3): 249–276. doi:10.1111/j.1095-8312.1983.tb01876.x. ISSN 0024-4066.
- Škaloud, Pavel; Steinová, Jana; Řídká, Tereza; Vančurová, Lucie; Peksa, Ondřej (4 May 2015). "Assembling the challenging puzzle of algal biodiversity: species delimitation within the genusAsterochloris(Trebouxiophyceae, Chlorophyta)". Journal of Phycology. 51 (3): 507–527. doi:10.1111/jpy.12295. ISSN 0022-3646. PMID 26986666. S2CID 25190572.
- Ramel, Gordon. "Lichen Reproductive Structures". Archived from the original on 28 February 2014. Retrieved 22 August 2014.
- Murtagh GJ, Dyer PS, Crittenden PD (April 2000). "Sex and the single lichen". Nature. 404 (6778): 564. Bibcode:2000Natur.404..564M. doi:10.1038/35007142. PMID 10766229. S2CID 4425228.
- Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Dictionary of the Fungi (10th ed.). Wallingford: CABI. pp. 378–381. ISBN 978-0-85199-826-8.
- "Form and structure – Sticta and Dendriscocaulon". Australian National Botanic Gardens. Archived from the original on 28 April 2014. Retrieved 18 September 2014.
- Lutzoni, F.; Kauff, F.; Cox, C. J.; McLaughlin, D.; Celio, G.; Dentinger, B.; Padamsee, M.; Hibbett, D.; et al. (2004). "Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits". American Journal of Botany. 91 (10): 1446–1480. doi:10.3732/ajb.91.10.1446. PMID 21652303. S2CID 9432006.
- Sanders, W. B.; Moe, R. L.; Ascaso, C. (2004). "The intertidal marine lichen formed by the pyrenomycete fungus Verrucaria tavaresiae (Ascomycotina) and the brown alga Petroderma maculiforme (Phaeophyceae): thallus organization and symbiont interaction – NCBI". American Journal of Botany. 91 (4): 511–22. doi:10.3732/ajb.91.4.511. PMID 21653406.
- "Mutualisms between fungi and algae – New Brunswick Museum". Archived from the original on 18 September 2018. Retrieved 4 October 2018.
- Miller, Kathy Ann; Pérez-Ortega, Sergio. "Challenging the lichen concept: Turgidosculum ulvae – Cambridge". The Lichenologist. 50 (3): 341–356. doi:10.1017/S0024282918000117. Archived from the original on 7 October 2018. Retrieved 7 October 2018.
- Rybalka, N.; Wolf, M.; Andersen, R. A.; Friedl, T. (2013). "Congruence of chloroplast – BMC Evolutionary Biology – BioMed Central". BMC Evolutionary Biology. 13: 39. doi:10.1186/1471-2148-13-39. PMC 3598724. PMID 23402662.
- Lutzoni, Francois; Pagel, Mark; Reeb, Valerie (21 June 2001). "Major fungal lineages are derived from lichen symbiotic ancestors". Nature. 411 (6840): 937–940. Bibcode:2001Natur.411..937L. doi:10.1038/35082053. PMID 11418855. S2CID 4414913.
- Hawksworth, D.L. (1988). "The variety of fungal-algal symbioses, their evolutionary significance, and the nature of lichens". Botanical Journal of the Linnean Society. 96: 3–20. doi:10.1111/j.1095-8339.1988.tb00623.x. S2CID 49717944.
- Rikkinen J. (1995). "What's behind the pretty colors? A study on the photobiology of lichens". Bryobrothera. 4 (3): 375–376. doi:10.2307/3244316. JSTOR 3244316.
- Friedl, T.; Büdel, B. (1996). "Photobionts". In Nash III, T.H. (ed.). Lichen Biology. Cambridge: Cambridge University Press. pp. 9–26. doi:10.1017/CBO9780511790478.003. ISBN 978-0-521-45368-4.
- "Alan Silverside's Lichen Glossary (a-f), Alan Silverside". Archived from the original on 31 October 2014. Retrieved 10 October 2014.
- Hallenbeck, Patrick C. (18 April 2017). Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects. ISBN 9783319462615. Archived from the original on 4 October 2018. Retrieved 4 October 2018.
- Rikkinen, J. (2002). "Lichen Guilds Share Related Cyanobacterial Symbionts". Science. 297 (5580): 357. doi:10.1126/science.1072961. PMID 12130774. S2CID 35731669.
- O'Brien, H.; Miadlikowska, J.; Lutzoni, F. (2005). "Assessing host specialization in symbiotic cyanobacteria associated with four closely related species of the lichen fungus Peltigera". European Journal of Phycology. 40 (4): 363–378. doi:10.1080/09670260500342647. S2CID 4094256.
- Guzow-Krzeminska, B (2006). "Photobiont ?exibility in thelichen Protoparmeliopsis muralis as revealed by ITS rDNA analyses". Lichenologist. 38 (5): 469–476. doi:10.1017/s0024282906005068.
- Ohmura, Y.; Kawachi, M.; Kasai, F.; Watanabe, M. (2006). "Genetic combinations of symbionts in a vegetatively reproducing lichen, Parmotrema tinctorum, based on ITS rDNA sequences" (2006)". Bryologist. 109: 43–59. doi:10.1639/0007-2745(2006)109[0043:gcosia]2.0.co;2.
- Piercey-Normore (2006). "The lichen-forming asco-mycete Evernia mesomorpha associates with multiplegenotypes of Trebouxia jamesii". New Phytologist. 169 (2): 331–344. doi:10.1111/j.1469-8137.2005.01576.x. PMID 16411936.
- Lutzoni, François; Pagel, Mark; Reeb, Valérie (2001). "Major fungal lineages are derived from lichen symbiotic ancestors". Nature. 411 (6840): 937–940. Bibcode:2001Natur.411..937L. doi:10.1038/35082053. PMID 11418855. S2CID 4414913.
- "Lichens: Fossil Record" Archived 25 January 2010 at the Wayback Machine, University of California Museum of Paleontology.
- Speer BR, Waggoner B. "Fossil Record of Lichens". University of California Museum of Paleontology. Archived from the original on 25 January 2010. Retrieved 16 February 2010.
- Poinar Jr., GO. (1992). Life in Amber. Stanford University Press.
- Peterson EB. (2000). "An overlooked fossil lichen (Lobariaceae)". Lichenologist. 32 (3): 298–300. doi:10.1006/lich.1999.0257.
- Taylor, T. N.; Hass, H.; Remy, W.; Kerp, H. (1995). "The oldest fossil lichen". Nature. 378 (6554): 244. Bibcode:1995Natur.378..244T. doi:10.1038/378244a0. S2CID 4353572. Archived from the original on 11 January 2007.
- Taylor WA, Free CB, Helgemo R, Ochoada J (2004). "SEM analysis of spongiophyton interpreted as a fossil lichen". International Journal of Plant Sciences. 165 (5): 875–881. doi:10.1086/422129. S2CID 85382155.
- Jahren, A.H.; Porter, S.; Kuglitsch, J.J. (2003). "Lichen metabolism identified in Early Devonian terrestrial organisms". Geology. 31 (2): 99–102. Bibcode:2003Geo....31...99J. doi:10.1130/0091-7613(2003)031<0099:LMIIED>2.0.CO;2. ISSN 0091-7613. S2CID 49241170.
- Fletcher, B. J.; Beerling, D. J.; Chaloner, W. G. (2004). "Stable carbon isotopes and the metabolism of the terrestrial Devonian organism Spongiophyton". Geobiology. 2 (2): 107–119. doi:10.1111/j.1472-4677.2004.00026.x.
- Edwards D; Axe L (2012). "Evidence for a fungal affinity for Nematasketum, a close ally of Prototaxites". Botanical Journal of the Linnean Society. 168: 1–18. doi:10.1111/j.1095-8339.2011.01195.x.
- Retallack G.J.; Landing, E. (2014). "Affinities and architecture of Devonian trunks of Prototaxites loganii". Mycologia. 106 (6): 1143–1156. doi:10.3852/13-390. PMID 24990121. S2CID 20013625.
- Karatygin IV; Snigirevskaya NS; Vikulin SV. (2009). "The most ancient terrestrial lichen Winfrenatia reticulata : A new find and new interpretation". Paleontological Journal. 43 (1): 107–114. doi:10.1134/S0031030109010110. S2CID 85262818.
- Karatygin IV, Snigirevskaya NS, Vikulin SV (2007). "Two types of symbiosis with participation of Fungi from Early Devonian Ecosystems". XV Congress of European Mycologists, Saint Petersburg, Russia, September 16–21, 2007. 1 (1): 226. Archived from the original on 24 April 2013. Retrieved 18 February 2011.
- "Lichens Are Way Younger Than Scientists Thought – Likely Evolved Millions of Years After Plants". 15 November 2019. Archived from the original on 18 December 2019. Retrieved 18 November 2019.
- Hansen, Karen; Perry, Brian A.; Dranginis, Andrew W.; Pfister, Donald H. (May 2013). "A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits". Molecular Phylogenetics and Evolution. 67 (2): 311–335. doi:10.1016/j.ympev.2013.01.014. ISSN 1055-7903. PMID 23403226.
- Schoch CL; Sung GH; López-Giráldez F; Townsend JP; Miadlikowska J; Hofstetter V; Robbertse B; Matheny PB; et al. (2009). "The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits". Syst. Biol. 58 (2): 224–239. doi:10.1093/sysbio/syp020. PMID 20525580.
- Gargas, A; Depriest, PT; Grube, M; Tehler, A (1995). "Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny". Science. 268 (5216): 1492–1495. Bibcode:1995Sci...268.1492G. doi:10.1126/science.7770775. PMID 7770775. S2CID 563601.
- Honegger R. (1998). "The lichen symbiosis – what is so spectacular about it?" (PDF). Lichenologist. 30 (3): 193–212. doi:10.1017/s002428299200015x. Archived (PDF) from the original on 26 April 2019. Retrieved 30 January 2019.
- Wedin M, Döring H, Gilenstam G (2004). "Saprotrophy and lichenization as options for the same fungl species on different substrata: environmental plasticity and fungal lifestyles in the Strictis-Conotrema complex". New Phytologist. 16 (3): 459–465. doi:10.1111/j.1469-8137.2004.01198.x.
- Yuan X, Xiao S, Taylor TN (2005). "Lichen-like symbiosis 600 million years ago". Science. 308 (5724): 1017–1020. Bibcode:2005Sci...308.1017Y. doi:10.1126/science.1111347. PMID 15890881. S2CID 27083645.
- Retallack G.J. (2015). "Acritarch evidence of a late Precambrian adaptive radiation of Fungi" (PDF). Botanica Pacifica. 4 (2): 19–33. doi:10.17581/bp.2015.04203. Archived (PDF) from the original on 22 December 2016. Retrieved 22 December 2016.
- Retallack GJ. (2007). "Growth, decay and burial compaction of Dickinsonia, an iconic Ediacaran fossil". Alcheringa: An Australasian Journal of Palaeontology. 31 (3): 215–240. doi:10.1080/03115510701484705. S2CID 17181699.
- Retallack GJ. (1994). "Were the Ediacaran Fossils Lichens?". Paleobiology. 20 (4): 523–544. doi:10.1017/s0094837300012975. JSTOR 2401233.
- Switek B (2012). "Controversial claim puts life on land 65 million years early". Nature. doi:10.1038/nature.2012.12017. S2CID 130305901. Archived from the original on 1 January 2013. Retrieved 2 January 2013.
- Retallack, G.J.; Dunn, K.L.; Saxby, J. (2015). "Problematic Mesoproterozoic fossil Horodyskia from Glacier National Park, Montana, USA". Precambrian Research. 226: 125–142. Bibcode:2013PreR..226..125R. doi:10.1016/j.precamres.2012.12.005.
- Retallack, G.J.; Krull, E.S.; Thackray, G.D.; Parkinson, D. (2013). "Problematic urn-shaped fossils from a Paleoproterozoic (2.2 Ga) paleosol in South Africa". Precambrian Research. 235: 71–87. Bibcode:2013PreR..235...71R. doi:10.1016/j.precamres.2013.05.015.
- Lücking, Robert; Nelsen, Matthew P. (2018), "Ediacarans, Protolichens, and Lichen-Derived Penicillium", Transformative Paleobotany, Elsevier, pp. 551–590, doi:10.1016/b978-0-12-813012-4.00023-1, ISBN 978-0-12-813012-4, retrieved 14 November 2020
- In the Race to Live on Land, Lichens Didn't Beat Plants – The New York Times
- "Pollution, The Plant Underworld". Australian National Botanic Gardens. Archived from the original on 17 February 2014. Retrieved 10 October 2014.
- Chen, Jie; Blume, Hans-Peter; Beyer, Lothar (2000). "Weathering of rocks induced by lichen colonization — a review" (PDF). CATENA. 39 (2): 121. doi:10.1016/S0341-8162(99)00085-5. Archived (PDF) from the original on 2 April 2015. Retrieved 21 March 2015.
- Jones, Clive G.; Shachak, Moshe (1990). "Fertilization of the desert soil by rock-eating snails". Nature. 346 (6287): 839. Bibcode:1990Natur.346..839J. doi:10.1038/346839a0. S2CID 4311333.
- Walker, T. R. (2007). "Lichens of the boreal forests of Labrador, Canada: A checklist". Evansia. 24 (3): 85–90. doi:10.1639/0747-9859-24.3.85. S2CID 129100097.
- Oksanen, I. (2006). "Ecological and biotechnological aspects of lichens". Applied Microbiology and Biotechnology. 73 (4): 723–734. doi:10.1007/s00253-006-0611-3. PMID 17082931. S2CID 12172616.
- Lawrey, James D. (1994). "Lichen Allelopathy: A Review". In Inderjit; K. M. M. Dakshini; Frank A. Einhellig (eds.). Allelopathy. Organisms, Processes, and Applications. ACS Symposium Series. 582. American Chemical Society. pp. 26–38. doi:10.1021/bk-1995-0582.ch002. ISBN 978-0-8412-3061-3.
- Nash III, Thomas H. (2008). "Lichen sensitivity to air pollution". In Nash III, T.H. (ed.). Lichen Biology (2nd ed.). Cambridge: Cambridge University Press. pp. 299–314. doi:10.1017/CBO9780511790478.016. ISBN 978-0-521-69216-8.
- Knops, J.M.H.; Nash, T. H. III; Boucher, V.L.; Schlesinger, W.H. (1991). "Mineral cycling and epiphytic lichens: Implications at the ecosystem level". Lichenologist. 23 (3): 309–321. doi:10.1017/S0024282991000452.
- Halonen P, Hyvarinen M, Kauppi M (1993). "Emission related and repeated monitoring of element concentrations in the epiphytic lichen Hypogymnia physodes in a coastal area, western Finland". Annales Botanici Fennici. 30: 251–261.
- Walker T. R.; Pystina T. N. (2006). "The use lichens to monitor terrestrial pollution and ecological impacts caused by oil and gas industries in the Pechora Basin, NW Russia". Herzogia. 19: 229–238.
- Walker T. R.; Crittenden P. D.; Young S. D.; Prystina T. (2006). "An assessment of pollution impacts due to the oil and gas industries in the Pechora basin, north-eastern European Russia". Ecological Indicators. 6 (2): 369–387. doi:10.1016/j.ecolind.2005.03.015.
- Walker T. R.; Crittenden P. D.; Young S. D. (2003). "Regional variation in the chemical composition of winter snowpack and terricolous lichens in relation to sources of acid emissions in the Usa River Basin, northeastern European Russia". Environmental Pollution. 125 (3): 401–412. doi:10.1016/s0269-7491(03)00080-0. PMID 12826418.
- Weiss-Penzias, Peter S.; Bank, Michael S.; Clifford, Deana L.; Torregrosa, Alicia; Zheng, Belle; Lin, Wendy; Wilmers, Christopher C. (26 November 2019). "Marine fog inputs appear to increase methylmercury bioaccumulation in a coastal terrestrial food web". Scientific Reports. 9 (1): 17611. Bibcode:2019NatSR...917611W. doi:10.1038/s41598-019-54056-7. PMC 6879473. PMID 31772229.
- Hogan, C. Michael (2010). "Abiotic factor". Encyclopedia of Earth. Washington, D.C.: National Council for Science and the Environment. Archived from the original on 8 June 2013. Retrieved 27 October 2013.
- Beltman IH, de Kok LJ, Kuiper PJC, van Hasselt PR (1980). "Fatty acid composition and chlorophyll content of epiphytic lichens and a possible relation to their sensitivity to air pollution". Oikos. 35 (3): 321–326. doi:10.2307/3544647. JSTOR 3544647.
- Emmerich R, Giez I, Lange OL, Proksch P (1993). "Toxicity and antifeedant activity of lichen compounds against the polyphagous herbivorous insect Spodoptera littoralis". Phytochemistry. 33 (6): 1389–1394. doi:10.1016/0031-9422(93)85097-B.
- Beschel RE (1950). "Flecten als altersmasstab Rezenter morainen". Zeitschrift für Gletscherkunde und Glazialgeologie. 1: 152–161.
- Curry, R. R. (1969) "Holocene climatic and glacial history of the central Sierra Nevada, California", pp. 1–47, Geological Society of America Special Paper, 123, S. A. Schumm and W. C. Bradley, eds.
- Sowers, J. M., Noller, J. S., and Lettis, W. R. (eds.) (1997) Dating and Earthquakes: Review of Quaternary Geochronology and its Application to Paleoseismology. U.S. Nuclear Regulatory Commission, NUREG/CR 5562.
- Innes, J. L. (1985). "Lichenometry". Progress in Physical Geography. 9 (2): 187. doi:10.1177/030913338500900202. S2CID 220949784.
- Cappitelli, Francesca; Sorlini, Claudia (2008). "Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage". Applied and Environmental Microbiology. 74 (3): 564–569. doi:10.1128/AEM.01768-07. PMC 2227722. PMID 18065627.
- Gadd, Geoffrey Michael (March 2010). "Metals, minerals and microbes: geomicrobiology and bioremediation". Microbiology. 156 (Pt 3): 609–643. doi:10.1099/mic.0.037143-0. PMID 20019082.
- Johnson, Cristopher J; Bennet, James P; Biro, Steven M; Duque-Velasquez, Juan Camilo; Rodriguez, Cynthia M.; Bessen, Richard A; Rocke, Tonie E (May 2011). Bartz, Jason C (ed.). "Degradation of the Disease-Associated Prion Protein by a Serine Protease from Lichens". PLOS ONE. 6 (5): e19836. Bibcode:2011PLoSO...619836J. doi:10.1371/journal.pone.0019836. PMC 3092769. PMID 21589935.
- Rodriguez, Cynthia M; Bennet, James P; Johnson, Cristopher J (1 January 2012). "Lichens: Unexpected anti-prion agents?". Prion. 6 (1): 11–16. doi:10.4161/pri.6.1.17414. PMC 3338958. PMID 22453171.
- Bennet, James P; Rodriuguez, Cynthia M; Johnson, Cristopher (July 2012). "Prion protein degradation by lichens of the genus Cladonia". The Lichenologist. 44 (4): 523–531. doi:10.1017/S0024282912000102.
- Casselman, Karen Leigh; Dean, Jenny (1999). Wild color: [the complete guide to making and using natural dyes]. New York: Watson-Guptill Publications. ISBN 978-0-8230-5727-6.
- Muller, K (2001). "Pharmaceutically Relevant Metabolites from Lichens". Applied Microbiology and Biotechnology. 56 (1–2): 9–10. doi:10.1007/s002530100684. PMID 11499952. S2CID 11958438.
- Morton, E.; Winters, J. and Smith, L. (2010). "An Analysis of Antiseptic and Antibiotic Properties of Variously Treated Mosses and Lichens" Archived 20 August 2017 at the Wayback Machine. University of Michigan Biological Station
- Bustinza, F. (1952). "Antibacterial Substances from Lichens". Economic Botany. 6 (4): 402–406. doi:10.1007/bf02984888. S2CID 39335883.
- "Themodelrailroader.com". Archived from the original on 15 October 2014. Retrieved 10 October 2014.
- Thus explained by Rabbi Enoch Zundel ben Joseph, in his commentary Etz Yosef ("Tree of Joseph"), on Sefer Midrash Rabbah, vol. 2, New York 1987, s.v. Ruth Rabba 6:3
- Zohar Amar and Yaron Serri, The Land of Israel and Syria as Described by Al-Tamimi, Ramat-Gan 2004, pp. 56, 108–109 ISBN 965-226-252-8 (Hebrew)
- Honegger R. (2000). "Simon Schwender (1829–1919) and the dual hypothesis in lichens". Bryologist. 103 (2): 307–313. doi:10.1639/0007-2745(2000)103[0307:SSATDH]2.0.CO;2. ISSN 0007-2745. JSTOR 3244159.
- Treub, Melchior (1873) Onderzoekingen over de natuur der lichenen. Dissertation Leiden University.
- Yong, Ed (21 July 2016), "How a guy from a Montana trailer park overturned 150 years of biology", The Atlantic, archived from the original on 23 July 2017, retrieved 23 July 2017.
Further reading
- Jorgensen, Per M., and Lücking, Robert (April 2018). "The 'Rustici Pauperrimi': A Linnaean Myth about Lichens Rectified". The Linnean 34(1), pp. 9–12.
External links
| Wikimedia Commons has media related to Lichens. |
| Look up lichen in Wiktionary, the free dictionary. |
- LIAS light: an online interactive identification key for the lichen species of the World
- University of California Museum of Paleontology microscopic image of cross section of crustose or squamulose lichen
- Earth Life Web – Schematic drawings of internal lichen structures for various growth forms
- University of Sydney lichen biology
- Memorial University's NL Nature project, focusing primarily on lichens
- Fungi that discovered agriculture
- The British Lichen Society
