Megapode
The megapodes, also known as incubator birds or mound-builders, are stocky, medium-large, chicken-like birds with small heads and large feet in the family Megapodiidae. Their name literally means "large foot" and is a reference to the heavy legs and feet typical of these terrestrial birds. All are browsers, and all but the malleefowl occupy wooded habitats. Most are brown or black in color. Megapodes are superprecocial, hatching from their eggs in the most mature condition of any bird. They hatch with open eyes, bodily coordination and strength, full wing feathers, and downy body feathers, and are able to run, pursue prey, and in some species, fly on the same day they hatch.[1]
| Megapode | |
|---|---|
 | |
| Australian brushturkey (Alectura lathami) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Galliformes |
| Family: | Megapodiidae Lesson, 1831 |
| Genera | |
| |
Description
Megapodes are medium-sized to large terrestrial birds with large legs and feet with sharp claws. The largest members of the clade are the species of Alectura and Talegalla. The smallest are the Micronesian scrubfowl (Megapodius laperouse) and the Moluccan scrubfowl (Eulipoa wallacei). They have small heads, short beaks, and rounded and large wings. Their flying abilities vary within the clade. They present the hallux at the same level of the other toes just like the species of the clade Cracidae. The other Galliformes have their halluces raised above the level of the front toes.[2]
Distribution and habitat
Megapodes are found in the broader Australasian region, including islands in the western Pacific, Australia, New Guinea, and the islands of Indonesia east of the Wallace Line, but also the Andaman and Nicobar Islands in the Bay of Bengal. The distribution of the family has contracted in the Pacific with the arrival of humans, and a number of island groups such as Fiji, Tonga, and New Caledonia have lost many or all of their species. Raoul Island, a New Zealand territory and the main island of the Kermadec Islands, may also have once had a species of megapode, based on settler accounts.[3]
Behaviour and ecology
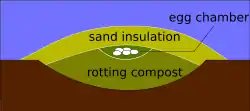
Megapodes are mainly solitary birds that do not incubate their eggs with their body heat as other birds do, but bury them. Their eggs are unusual in having a large yolk, making up 50–70% of the egg weight.[4] The birds are best known for building massive nest mounds of decaying vegetation, which the male attends, adding or removing litter to regulate the internal heat while the eggs develop. However, some bury their eggs in other ways; there are burrow-nesters which use geothermal heat, and others which simply rely on the heat of the sun warming sand. Some species vary their incubation strategy depending on the local environment.[3] Although the Australian brushturkey was thought to exhibit temperature-dependent sex determination, this was later proven false;[5] temperature does, however, affect embryo mortality and resulting offspring sex ratios. The nonsocial nature of their incubation raises questions as to how the hatchlings come to recognise other members of their species, which is due to imprinting in other members of the order Galliformes. Research suggests an instinctive visual recognition of specific movement patterns is made by the individual species of megapode.[6]
Megapode chicks do not have an egg tooth; they use their powerful claws to break out of the egg, and then tunnel their way up to the surface of the mound, lying on their backs and scratching at the sand and vegetable matter. Similar to other superprecocial birds, they hatch fully feathered and active, already able to fly and live independently from their parents.[4] In megapodes superprecociality apparently evolved secondarily from brooding and at least loose parental care as more typical in Galliformes.[7] Eggs previously assigned to Genyornis have been reassigned to giant megapode species. Some dietary and chronological data previously assigned to dromornithids may instead be assigned to the giant megapodes.[8]
Megapodes share some similarities to the extinct enantiornithes in terms of their superprecocial life cycle, though also several differences.[9]
Species
The more than 20 living species are placed in seven genera. Although the evolutionary relationships between the Megapodiidae are especially uncertain, the morphological groups are clear:[10]


Phylogeny
Living Megapodiidae, based on the work by John Boyd:[11]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Taxonomy
- Genus †Mwalau Worthy et al. 2015
- †Mwalau walterlinii Worthy et al. 2015 (Vanuatu)
- Genus †Ngawupodius Boles & Ivison 1999
- †Ngawupodius minya Boles & Ivison 1999
- Scrubfowl group
- Genus: Macrocephalon
- Maleo, Macrocephalon maleo
- Genus: Eulipoa (sometimes included in Megapodius)
- Moluccan megapode, Eulipoa wallacei.
- Genus: Megapodius
- Tongan megapode, Megapodius pritchardii
- Micronesian megapode, Megapodius laperouse
- Marianas Island megapode, Megapodius laperouse laperouse
- Palau Island megapode, Megapodius laperouse senex
- Nicobar megapode, Megapodius nicobariensis
- Philippine megapode, Megapodius cumingii
- Sula megapode, Megapodius bernsteinii
- Tanimbar megapode, Megapodius tenimberensis
- Dusky megapode, Megapodius freycinet
- Forsten's megapode, Megapodius (freycinet) forstenii
- Biak scrubfowl, Megapodius geelvinkianus
- Melanesian megapode, Megapodius eremita
- Vanuatu megapode, Megapodius layardi
- New Guinea scrubfowl, Megapodius affinis
- Orange-footed scrubfowl, Megapodius reinwardt
- †Pile-builder scrubfowl, Megapodius molistructor Balouet & Olson 1989
- †Viti Levu scrubfowl, Megapodius amissus Worthy 2000
- †Consumed scrubfowl, Megapodius alimentum Steatman 1989a
- †M. andamanensis Walter 1980 nomen dubium [oospecies]
- †M. burnabyi Gray 1861 nomen dubium [oospecies]
- †Raoul Island scrubfowl, M. sp.
- †'Eua scrubfowl, (small-fooed megapode), M. sp.
- †Lifuka scrubfowl, M. sp.
- †Stout Tongan megapode, M. sp.
- †Large Vanuatu megapode, M. sp.
- †Large Solomon Islands, M. sp.
- †New Caledonia megapode, M. sp.
- †Loyalty megapode, M. sp.
- †New Ireland scrubfowl (large Bismarck's megapode), M. sp.
- Genus: Macrocephalon
- Malleefowl, group
- Genus: Leipoa
- Malleefowl, Leipoa ocellata
- Genus: Leipoa
- Brushturkey group
- Genus: Alectura
- Australian brushturkey, Alectura lathami
- Genus: Aepypodius
- Wattled brushturkey, Aepypodius arfakianus
- Waigeo brushturkey, Aepypodius bruijnii
- Genus: Talegalla
- Red-billed brushturkey, Talegalla cuvieri
- Black-billed brushturkey, Talegalla fuscirostris
- Collared brushturkey, Talegalla jobiensis
- Genus: †Progura
- Progura gallinacea - Queensland, Pleistocene
- Progura campestris - SA, Pleistocene
- Genus: †Latagallina
- Latagallina naracoortensis formerly Progura naracoortensis - NSW, SA, Pleistocene
- Latagallina olsoni - SA, Pleistocene
- Genus: Alectura
- Incertae sedis
- Genus: †Garrdimalga
- Garrdimalga mcnamarai - SA, Pleistocene
- Genus: †Garrdimalga
See also
- List of recently extinct birds
- Late Quaternary prehistoric birds
- List of fossil bird genera
References
- Starck, J.M., Ricklefs, R.E. (1998). Avian Growth and Development. Evolution within the altricial precocial spectrum. New York: Oxford University Press. ISBN 978-0-19-510608-4.CS1 maint: multiple names: authors list (link)
- del Hoyo, J.; Elliott, A.; Sargatal, J. (1994). Handbook of the Birds of the World. Volume 2: New World Vultures to Guineafowl. Lynx Edicions. ISBN 978-84-87334-15-3.
- Steadman D, (2006). Extinction and Biogeography in Tropical Pacific Birds, University of Chicago Press. ISBN 978-0-226-77142-7
- Starck, J.M. & Sutter E. (2000). "Patterns of growth and heterochrony in moundbuilders (MEgapodiidae) and fowl (Phasianidae)". Journal of Avian Biology. 31 (4): 527–47. doi:10.1034/j.1600-048x.2000.310413.x.
- Göth, Ann; Booth, David T (22 March 2005). "Temperature-dependent sex ratio in a bird". Biology Letters. 1 (1): 31–33. doi:10.1098/rsbl.2004.0247. PMC 1629050. PMID 17148121.
- Göth, A., Evans, C.S. (2004). "Social responses without early experience: Australian brush-turkey chicks use specific visual cues to aggregate with conspecifics". Journal of Experimental Biology. 207 (13): 2199–2208. doi:10.1242/jeb.01008. PMID 15159424.CS1 maint: multiple names: authors list (link)
- Harris, R. B.; Birks, S. M.; Leaché, A. D. (2014). "Incubator birds: biogeographical origins and evolution of underground nesting in megapodes (Galliformes: Megapodiidae)". Journal of Biogeography. 41: 2045–2056. doi:10.1111/jbi.12357.
- A case of mistaken identity for Australia’s extinct big bird
- https://www.researchgate.net/publication/338355119_First_report_of_immature_feathers_in_juvenile_enantiornithines_from_the_Early_Cretaceous_Jehol_avifauna; "These feather traces and the plumage in HPG-15-1 strongly suggest that members of the Enantiornithes were born fully fledged and capable of flight soon after hatching, somewhat resembling the super-precocial megapodes, the only group of neornithines in which neonates are similarly born fledged and capable of flight (Zhou and Zhang, 2004; Jones and Göth, 2008; Xing et al., 2017). Megapodes do not fly immediately, requiring nearly two days to dig themselves out of their mounds during which they preen off their feather sheaths and let their feathers dry (Jones and Göth, 2008). Similarly, hatchling enantiornithines would have had to wait until their feather sheaths were removed and their feathers dry before attempting flight. Although ecological and behavioural differences clearly exist between enantiornithines and megapodes (e.g., enantiornithines were arboreal and not mound-nesters), megapodes represent the precocial extreme in extant neornithines and thus the closest analogue for enantiornithine development, for which all evidence indicates a form of extreme precociality (Elzanowski, 1981; Zhou and Zhang, 2004; Xing et al., 2017). "
- Birks, S.M. & S.V. Edwards (2002). A phylogeny of the megapodes (Aves: Megapodiidae) based on nuclear and mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 23. pp. 408–21.
- Taxonomy in Flux Boyd, John (2007). "Megapodiidae" (PDF). Retrieved 30 August 2016.
External links
| Wikimedia Commons has media related to Megapodiidae. |
- Mound-builders videos, photos & sounds on the Internet Bird Collection
- Photograph of a nest mound of M. tenimberensis from the Oriental Bird Club