Mixture
In chemistry, a mixture is a material made up of two or more different substances which are not chemically combined.[1] A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids.[2][3]
Mixtures are one product of mechanically blending or mixing chemical substances such as elements and compounds, without chemical bonding or other chemical change, so that each ingredient substance retains its own chemical properties and makeup.[4] Despite the fact that there are no chemical changes to its constituents, the physical properties of a mixture, such as its melting point, may differ from those of the components. Some mixtures can be separated into their components by using physical (mechanical or thermal) means. Azeotropes are one kind of mixture that usually poses considerable difficulties regarding the separation processes required to obtain their constituents (physical or chemical processes or, even a blend of them).[5][6][7]
Characteristics of mixtures
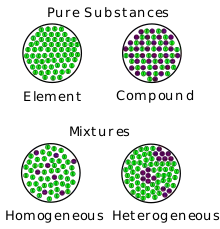
Mixtures can be characterized by being separable by mechanical means e.g. heat, filtration, gravitational sorting, centrifugation etc.[8] Mixtures can be either homogeneous or heterogeneous': a mixture in which constituents are distributed uniformly is called homogeneous, such as salt in water, otherwise it is called heterogeneous, such as sand in water.
One example of a mixture is air. Air is a homogeneous mixture of the gaseous substances nitrogen, oxygen, and smaller amounts of other substances. Salt, sugar, and many other substances dissolve in water to form homogeneous mixtures. A homogeneous mixture in which there is both a solute and solvent present is also a solution. Mixtures can have any amounts of ingredients.
Mixtures are unlike chemical compounds, because:
- The substances in a mixture can be separated using physical methods such as filtration, freezing, and distillation.
- There is little or no energy change when a mixture forms (see Enthalpy of mixing).
- Mixtures have variable compositions, while compounds have a fixed, definite formula.
- When mixed, individual substances keep their properties in a mixture, while if they form a compound their properties can change.[9]
The following table shows the main properties of the three families of mixtures and examples of the three types of mixture.
| Dispersion medium (mixture phase) | Dissolved or dispersed phase | Solution | Colloid | Suspension (coarse dispersion) |
|---|---|---|---|---|
| Gas | Gas | Gas mixture: air (oxygen and other gases in nitrogen) | None | None |
| Liquid | None | Liquid aerosol:[10] fog, mist, vapor, hair sprays | Spray | |
| Solid | None | Solid aerosol:[10] smoke, ice cloud, air particulates | Dust | |
| Liquid | Gas | Solution: oxygen in water | Liquid foam: whipped cream, shaving cream | Sea foam, beer head |
| Liquid | Solution: alcoholic beverages | Emulsion: milk, mayonnaise, hand cream | Vinaigrette | |
| Solid | Solution: sugar in water | Liquid sol: pigmented ink, blood | Suspension: mud (soil, clay or silt particles are suspended in water), chalk powder suspended in water | |
| Solid | Gas | Solution: hydrogen in metals | Solid foam: aerogel, styrofoam, pumice | Foam: dry sponge |
| Liquid | Solution: amalgam (mercury in gold), hexane in paraffin wax | Gel: agar, gelatin, silicagel, opal | Wet sponge | |
| Solid | Solution: alloys, plasticizers in plastics | Solid sol: cranberry glass | Clay, silt, sand, gravel, granite |
Homogeneous and heterogeneous mixtures
A homogeneous mixture has the same proportions of its components throughout any given sample and is also referred to as a solution. Conversely, a heterogeneous mixture has components of which proportions vary throughout the sample. "Homogeneous" and "heterogeneous" are not absolute terms, but are dependent on context and the size of the sample.
In chemistry, if the volume of a homogeneous suspension is divided in half, the same amount of material is suspended in both halves of the substance. An example of a homogeneous mixture is air.
In physical chemistry and materials science this refers to substances and mixtures which are in a single phase. This is in contrast to a substance that is heterogeneous.[11]
Solution
A solution is a special type of homogeneous mixture where the ratio of solute to solvent remains the same throughout the solution and the particles are not visible with the naked eye, even if homogenized with multiple sources. In solutions, solutes will not settle out after any period of time and they can't be removed by physical methods, such as a filter or centrifuge.[12] As a homogeneous mixture, a solution has one phase (solid, liquid, or gas), although the phase of the solute and solvent may initially have been different (e.g., salt water).
Gases
Air can be more specifically described as a gaseous solution (oxygen and other gases dissolved in the major component, nitrogen). Since interactions between molecules play almost no role, dilute gases form trivial solutions. In part of the literature, they are not even classified as solutions. In gas, intermolecular space is the greatest—and intermolecular force of attraction is least. Some examples can be oxygen, hydrogen, or nitrogen.
Distinguishing between mixture types
Making a distinction between homogeneous and heterogeneous mixtures is a matter of the scale of sampling. On a coarse enough scale, any mixture can be said to be homogeneous, if the entire article is allowed to count as a "sample" of it. On a fine enough scale, any mixture can be said to be heterogeneous, because a sample could be as small as a single molecule. In practical terms, if the property of interest of the mixture is the same regardless of which sample of it is taken for the examination used, the mixture is homogeneous.
Gy's sampling theory quantitavely defines the heterogeneity of a particle as:[13]
where , , , , and are respectively: the heterogeneity of the th particle of the population, the mass concentration of the property of interest in the th particle of the population, the mass concentration of the property of interest in the population, the mass of the th particle in the population, and the average mass of a particle in the population.
During sampling of heterogeneous mixtures of particles, the variance of the sampling error is generally non-zero.
Pierre Gy derived, from the Poisson sampling model, the following formula for the variance of the sampling error in the mass concentration in a sample:
in which V is the variance of the sampling error, N is the number of particles in the population (before the sample was taken), q i is the probability of including the ith particle of the population in the sample (i.e. the first-order inclusion probability of the ith particle), m i is the mass of the ith particle of the population and a i is the mass concentration of the property of interest in the ith particle of the population.
The above equation for the variance of the sampling error is an approximation based on a linearization of the mass concentration in a sample.
In the theory of Gy, correct sampling is defined as a sampling scenario in which all particles have the same probability of being included in the sample. This implies that q i no longer depends on i, and can therefore be replaced by the symbol q. Gy's equation for the variance of the sampling error becomes:
where abatch is that concentration of the property of interest in the population from which the sample is to be drawn and Mbatch is the mass of the population from which the sample is to be drawn.
Homogenization
See also
References
- Chemistry, International Union of Pure and Applied. "IUPAC Gold Book - mixture". goldbook.iupac.org. Retrieved 1 July 2019.
- Whitten K.W., Gailey K. D. and Davis R. E. (1992). General chemistry, 4th Ed. Philadelphia: Saunders College Publishing. ISBN 978-0-03-072373-5.
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geography (2002). General chemistry: principles and modern applications (8th ed.). Upper Saddle River, N.J: Prentice Hall. ISBN 978-0-13-014329-7. LCCN 2001032331. OCLC 46872308.
- De Paula, Julio; Atkins, P. W. Atkins' Physical Chemistry (7th ed.). ISBN 978-0-19-879285-7.
- Alberts B.; et al. (2002). Molecular Biology of the Cell, 4th Ed. Garland Science. ISBN 978-0-8153-4072-0.
- Laidler K. J. (1978). Physical chemistry with biological applications. Benjamin/Cummings. Menlo Park. ISBN 978-0-8053-5680-9.
- Weast R. C., Ed. (1990). CRC Handbook of chemistry and physics. Boca Raton: Chemical Rubber Publishing Company. ISBN 978-0-8493-0470-5.
- Ashworth, William; Littl1, Charles E., eds. (2001). "Mixture". The Encyclopedia of Environmental Studies. Online publisher:Science Online. Facts On File, Inc.
- "Definition of mixture - Chemistry Dictionary". www.chemicool.com. Retrieved 30 November 2018.
- Everett, D. H. (23 July 1971). Manual of Symbols and Terminology for Physicochemical Quantities and Units. Appendix II Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Part I (PDF) (Report). London: International Union of Pure and Applied Chemistry: Division of Physical Chemistry. Archived (PDF) from the original on 28 October 2016. Retrieved 28 October 2016.
- Lew, Kristi (2009). "Homogeneous". Acids and Bases, Essential Chemistry. New York: Chelsea House Publishing. Online publisher: Science Online. Facts On File, Inc. ISBN 978-0-7910-9783-0. access date: 2010-01-01
- "Solution (chemistry)" (authors: William Ashworth and Charles E. Little)
|chapter-format=requires|chapter-url=(help). Encyclopedia of Environmental Studies, New Edition. Online publisher:Science Online. Facts On File, Inc. 2001. access date: 2010-01-01 - Gy, P (1979). Sampling of Particulate Materials: Theory and Practice. Amsterdam: Elsevier.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "mixture". doi:10.1351/goldbook.M03949