Multituberculata
Multituberculata (commonly known as multituberculates, named for the multiple tubercles of their teeth) is an extinct taxon of rodent-like allotherian mammals that existed for approximately 166 million years,[3][4] the longest fossil history of any mammal lineage. They eventually declined from the late Paleocene onwards, disappearing from the known fossil record in the late Eocene,[5] though gondwanatheres lived into the Miocene, and may have been multituberculates. More than 200 species are known, ranging from mouse-sized to beaver-sized. These species occupied a diversity of ecological niches, ranging from burrow-dwelling to squirrel-like arborealism to jerboa-like hoppers.[6][7] Multituberculates are usually placed as crown mammals outside either of the two main groups of living mammals—Theria, including placentals and marsupials, and Monotremata[8]—but closer to Theria than to monotremes.[9][10] Nonetheless, at least one study found a potential status as sister taxa to monotremes/Australosphenida.[11]
| Multituberculates | |
|---|---|
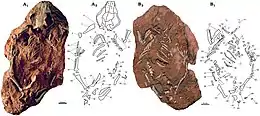 | |
| Skeleton of Catopsbaatar | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Subclass: | Theriiformes |
| Order: | †Multituberculata Cope, 1884 |
| Suborders | |
Description

The multituberculates had a cranial and dental anatomy superficially similar to rodents such as mice and rats, with cheek-teeth separated from the chisel-like front teeth by a wide tooth-less gap (the diasteme). Each cheek-tooth displayed several rows of small cusps (or tubercles, hence the name) that operated against similar rows in the teeth of the jaw; the exact homology of these cusps to therian ones is still a matter of debate. Unlike rodents, which have ever-growing teeth, multituberculates underwent dental replacement patterns typical to most mammals (though in at least some species the lower incisors continued to erupt long after the root's closure).[12] Multituberculates are notable for the presence of a massive fourth lower premolar, the plagiaulacoid; other mammals, like Plesiadapiformes and diprotodontian marsupials, also have similar premolars in both upper and lower jaws, but in multituberculates this tooth is massive and the upper premolars aren't modified this way. In basal multituberculates all three lower premolars were plagiaulacoids, increasing in size posteriorly, but in Cimolodonta only the fourth lower premolar remained, with the third one remaining only as a vestigial peg-like tooth,[12] and in several taxa like gondwanatherians and taeniolabidoideans, the plagiaulacoid disappeared entirely or was reconverted into a molariform tooth.[13]
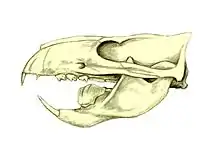
Unlike rodents and similar therians, multituberculates had a palinal jaw stroke (front-to-back), instead of a propalinal (back-to-front) or transverse (side-to-side) one; as a consequence, their jaw musculature and cusp orientation is radically different.[8][12] Palinal jaw strokes are almost entirely absent in modern mammals (with the possible exception of the dugong[14]), but are also present in haramiyidans, argyrolagoideans and tritylodontids, the former historically united with multituberculates on that basis. Multituberculate mastication is thought to have operated in a two stroke cycle: first, food held in place by the last upper premolar was sliced by the bladelike lower pre-molars as the dentary moved orthally (upward). Then the lower jaw moved palinally, grinding the food between the molar cusp rows.[8][12]
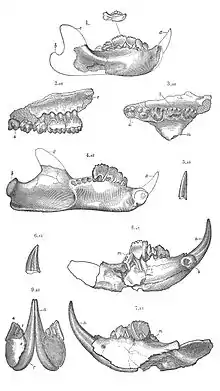
The structure of the pelvis in the Multituberculata suggests that they gave birth to tiny helpless, underdeveloped young, similar to modern marsupials, such as kangaroos.[6][12]
At least two lineages developed hypsodonty, in which tooth enamel extends beyond the gumline: lambdopsalid taeniolabidoideans[15] and sudamericid gondwanatheres.[16] The latter, having been around already during the Cretaceous, are the earliest known lineage of grazing mammals. A species from the Katsuyama Dinosaur Forest Geopark may offer an even earlier example of grass-eating adaptations as it dates from the Lower Cretaceous at about 120 million years.[17]
Studies published in 2018 demonstrated that multituberculates had relatively complex brains, some braincase regions even absent in therian mammals.[18]
Evolution
Multituberculates first appear in the fossil record during the Jurassic period, and then survived and even dominated for over one hundred million years, longer than any other order of mammaliforms, including placental mammals. The earliest known example is Rugosodon, a rodent-like omnivore living 160 million years ago in what is today China.
During the Cretaceous, the multituberculates radiated into a wide variety of morphotypes, including the squirrel-like arboreal ptilodonts. Most species of multituberculata appear to have been wiped out during the K-T event (the extinction of the dinosaurs), but they seem to have been among the first to recover and diversify again. The peculiar shape of their last lower premolar is their most outstanding feature. These teeth were larger and more elongated than the other cheek-teeth and had an occlusive surface forming a serrated slicing blade. Though it can be assumed that this was used for crushing seeds and nuts, it is believed that most small multituberculates also supplemented their diet with insects, worms, and fruits.[8] Tooth marks attributed to multituberculates are known on Champsosaurus fossils, indicating that at least some of these mammals were scavengers.[19]
A ptilodont that throve in North America was Ptilodus. Thanks to the well-preserved Ptilodus specimens found in the Bighorn Basin, Wyoming, we know that these multituberculates were able to abduct and adduct their big toes, and thus that their foot mobility was similar to that of modern squirrels, which descend trees head first.[8]

In Europe, another family of multituberculates were equally successful—the Kogaionidae, first discovered in Haţeg, Romania. They also developed an enlarged blade-like lower premolar. Hainina, the most successful genus, was originally believed to be a ptilodont. However, more detailed analysis of this genus revealed a smaller number of dental cusps and a retained fifth premolar—a unique combination of primitive and advanced features indicating that Hainina were related to some Jurassic genera and that enlarged, blade-like premolars were acquired independently in Europe and North America.[8]
Another group of multituberculates, the taeniolabids, were heavier and more massively built, indicating that they lived a fully terrestrial life. The largest specimens weighted probably as much as 100 kg, making them comparable in size to large rodents like Castoroides.[20] They reached their highest diversity in Asia during the late Cretaceous and Paleocene, which suggests that they originated from there.[8]
About 80 genera of Multituberculata are known, including Lambdopsalis, Ptilodus and Meniscoessus. In the northern hemisphere, during the late Cretaceous, more than half of typical land mammalian species were multituberculates. While most mammals — along with birds and other dinosaurs and most other types of life — were wiped out during the K-T event (the extinction of the dinosaurs 65 million years ago), a large proportion of the mammals that show up in the fossil record after the extinction are multituberculates.
The group went on to dominate land in the next twenty million years of the paleocene, but it appears that increasing competition from placental mammals drove them to extinction by the end of the Eocene, about 40 million years ago.
Classification

In their 2001 study, Kielan-Jaworowska and Hurum found that most multituberculates could be referred to two suborders: "Plagiaulacida" and Cimolodonta. The exception is the genus Arginbaatar, which shares characteristics with both groups.
"Plagiaulacida" is paraphyletic, representing the more primitive evolutionary grade and possibly the more derived Gondwanatheria. Its members are the more basal Multituberculata, though gondwanatherians are rather derived. Chronologically, they ranged from perhaps the middle Jurassic (unnamed material), until the lower Cretaceous. This group is further subdivided into three informal groupings: the allodontid line, the paulchoffatiid line, and the plagiaulacid line.
Gondwanatheria is a monophyletic group that was diverse in the Late Cretaceous of South America, India, Madagascar and possibly Africa and occurs onwards into the Cenozoic of South America and Antarctica. Though their identity as multituberculates has been disputed, most recent phylogenetic studies recover them as the sister group to cimolodonts. There are two major families, Ferugliotheriidae and Sudamericidae, with a few taxa like Greniodon and Groeberia being uncertainly placed. Patagonia is the youngest multituberculate known, occurring in the Miocene of Argentina.
Cimolodonta is, apparently, a natural (monophyletic) suborder. This includes the more derived Multituberculata, which have been identified from the lower Cretaceous to the Eocene. The superfamilies Djadochtatherioidea, Taeniolabidoidea, Ptilodontoidea are recognized, as is the Paracimexomys group. Additionally, there are the families Cimolomyidae, Boffiidae, Eucosmodontidae, Kogaionidae, Microcosmodontidae and the two genera Uzbekbaatar and Viridomys. More precise placement of these types awaits further discoveries and analysis.[21]
Taxonomy
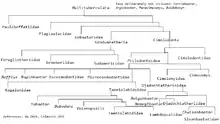
Based on the combined works of Mikko's Phylogeny Archive[22] and Paleofile.com.
Suborder †Plagiaulacida Simpson 1925
- Genus ?†Argillomys Cifelli, Gordon & Lipka 2013
- Species †Argillomys marylandensis Cifelli, Gordon & Lipka 2013
- Genus ?†Janumys Eaton & Cifelli 2001
- Species †Janumys erebos Eaton & Cifelli 2001
- Super family †Allodontoidea Marsh 1889
- Genus †?Glirodon Engelmann & Callison, 2001
- Species †G. grandis Engelmann & Callison, 2001
- Family †Arginbaataridae Hahn & Hahn, 1983
- Genus †Arginbaatar Trofimov, 1980
- Species †A. dmitrievae Trofimov, 1980
- Genus †Arginbaatar Trofimov, 1980
- Family †Zofiabaataridae Bakker, 1992
- Genus †Zofiabaatar Bakker & Carpenter, 1990
- Species †Z. pulcher Bakker & Carpenter, 1990
- Genus †Zofiabaatar Bakker & Carpenter, 1990
- Family †Allodontidae Marsh, 1889
- Genus †Passumys Cifelli, Davis & Sames 2014
- Species †Passumys angelli Cifelli, Davis & Sames 2014
- Genus †Ctenacodon Marsh, 1879
- Species †C. serratus Marsh, 1879
- Species †C. nanus Marsh, 1881
- Species †C. laticeps (Marsh, 1881) [Allodon laticeps Marsh 1881]
- Species †C. scindens Simpson, 1928
- Genus †Psalodon Simpson, 1926
- Genus †Passumys Cifelli, Davis & Sames 2014
- Genus †?Glirodon Engelmann & Callison, 2001
- Super family †Paulchoffatioidea Hahn 1969 sensu Hahn & Hahn 2003
- Genus ?†Mojo Hahn, LePage & Wouters 1987
- Species †Mojo usuratus Hahn, LePage & Wouters 1987
- Genus ?†Rugosodon Yuan et al., 2013
- Species †Rugosodon eurasiaticus Yuan et al., 2013
- Family †Pinheirodontidae Hahn & Hahn, 1999
- Genus †Bernardodon Hahn & Hahn, 1999
- Species †B. atlanticus Hahn & Hahn, 1999
- Species †B. sp. Hahn & Hahn, 1999
- Genus †Cantalera Badiola, Canudo & Cuenca-Bescos, 2008
- Species †Cantalera abadi Badiola, Canudo & Cuenca-Bescos, 2008
- Genus †Ecprepaulax Hahn & Hahn, 1999
- Species †E. anomala Hahn & Hahn, 1999
- Genus †Gerhardodon Kielan-Jaworowska & Ensom, 1992
- Species †G. purbeckensis Kielan-Jaworowska & Ensom, 1992
- Genus †Iberodon Hahn & Hahn, 1999
- Species †I. quadrituberculatus Hahn & Hahn, 1999
- Genus †Lavocatia Canudo & Cuenca-Bescós, 1996
- Species †L. alfambrensis Canudo & Cuenca-Bescós, 1996
- Genus †Pinheirodon Hahn & Hahn, 1999
- Species †P. pygmaeus Hahn & Hahn, 1999
- Species †P. vastus Hahn & Hahn, 1999
- Genus †Bernardodon Hahn & Hahn, 1999
- Family †Paulchoffatiidae Hahn, 1969
- Genus ?†Galveodon Hahn & Hahn, 1992
- Species †G. nannothus Hahn & Hahn, 1992
- Genus ?†Sunnyodon Kielan-Jaworowska & Ensom, 1992
- Species †S. notleyi Kielan-Jaworowska & Ensom, 1992
- subfamily †Paulchoffatiinae Hahn, 1971
- Genus †Paulchoffatia Kühne, 1961
- Species †P. delgador Kühne, 1961
- Genus †Pseudobolodon Hahn, 1977
- Genus †Henkelodon Hahn, 1987
- Species †H. naias Hahn, 1987
- Genus †Guimarotodon Hahn, 1969
- Species †G. leiriensis Hahn, 1969
- Genus †Meketibolodon (Hahn, 1978) Hahn, 1993
- Species †M. robustus (Hahn, 1978) Hahn, 1993 [Pseudobolodon robusutus Hahn 1978]
- Genus †Plesiochoffatia Hahn & Hahn, 1999 [Parachoffatia Hahn & Hahn 1998 non Mangold 1970]
- Species †P. thoas (Hahn & Hahn, 1998) Hahn & Hahn 1999 [Parachoffatia thoa Hahn & Hahn 1998]
- Species †P. peparethos (Hahn & Hahn, 1998) Hahn & Hahn 1999 [Parachoffatia peparethos Hahn & Hahn 1998]
- Species †P. staphylos (Hahn & Hahn, 1998) Hahn & Hahn 1999 [Parachoffatia staphylos Hahn & Hahn 1998]
- Genus †Xenachoffatia Hahn & Hahn, 1998
- Species †X. oinopion Hahn & Hahn, 1998
- Genus †Bathmochoffatia Hahn & Hahn, 1998
- Species †B. hapax Hahn & Hahn, 1998
- Genus †Kielanodon Hahn, 1987
- Species †K. hopsoni Hahn, 1987
- Genus †Meketichoffatia Hahn, 1993
- Species †M. krausei Hahn, 1993
- Genus †Renatodon Hahn, 2001
- Species †Renatodon amalthea Hahn, 2001
- Genus †Paulchoffatia Kühne, 1961
- Subfamily †Kuehneodontinae Hahn, 1971
- Genus †Kuehneodon Hahn, 1969
- Species †K. dietrichi Hahn, 1969
- Species †K. barcasensis Hahn & Hahn, 2001
- Species †K. dryas Hahn, 1977
- Species †K. guimarotensis Hahn, 1969
- Species †K. hahni Antunes, 1988
- Species †K. simpsoni Hahn, 1969
- Species †K. uniradiculatus Hahn, 1978
- Genus †Kuehneodon Hahn, 1969
- Genus ?†Galveodon Hahn & Hahn, 1992
- Genus ?†Mojo Hahn, LePage & Wouters 1987
- Super family †Plagiaulacoidea Ameghino, 1894
- Family †Plagiaulacidae Gill, 1872 sensu Kielan-Jaworowska & Hurum, 2001 [Bolodontidae Osborn 1887]
- Genus ?†Morrisonodon Hahn & Hahn, 2004
- Species †Morrisonodon brentbaatar (Bakker, 1998) Hahn & Hahn, 2004 [Ctenacodon brentbaatar Bakker, 1998]
- Genus †Plagiaulax Falconer, 1857
- Species †P. becklesii Falconer, 1857
- Species †P. dawsoni Woodward, 1891 [Plioprion dawsoni Woodward, 1891; Loxaulax dawsoni (Woodward, 1891) Sloan, 1979]
- Genus †Bolodon Owen, 1871 [Plioprion Cope, 1884]
- Species †B. crassidens Owen, 1871
- Species †B. falconeri Owen, 1871 [Pligiaulax falconeri Owen, 1871; Plioprion falconeri (Owen, 1871)]
- Species †B. hydei Cifelli, Davis & Sames, 2014
- Species †B. minor Falconer, 1857 [Pligiaulax minor Falconer, 1857; Plioprion minor (Falconer, 1857)]
- Species †B. osborni Simpson, 1928 [Plioprion osborni (Simpson, 1928); Ctenacodon osborni Simpson, 1928]
- Species ?†B. elongatus Simpson, 1928
- Genus ?†Morrisonodon Hahn & Hahn, 2004
- Family †Plagiaulacidae Gill, 1872 sensu Kielan-Jaworowska & Hurum, 2001 [Bolodontidae Osborn 1887]
- Family †Eobaataridae Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Genus †Eobaatar Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Species †E. clemensi Sweetman, 2009
- Species †E. hispanicus Hahn & Hahn, 1992
- Species †E. magnus Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Species †E. minor Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Species †E. pajaronensis Hahn & Hahn, 2001
- Genus †Hakusanobaatar Kusuhashi et al., 2008
- Species †H. matsuoi Kusuhashi et al., 2008
- Genus †Heishanobaatar Kusuhashi et al., 2010
- Species †H. triangulus Kusuhashi et al., 2010
- Genus †Iberica Badiola et al., 2011
- Species †Iberica hahni Badiola et al., 2011
- Genus †Liaobaatar Kusuhashi et al., 2009
- Species †L. changi Kusuhashi et al., 2009
- Genus †Loxaulax Simpson, 1928 [Parendotherium Crusafont Pairó & Adrover, 1966]
- Species †L. valdensis (Woodward, 1911) Simpson, 1928[Dipriodon valdensis Woodward, 1911]
- Species †L. herreroi (Crusafont Pairó & Adrover, 1966) [Parendotherium herreroi Crusafont Pairó & Adrover 1966]
- Genus †Monobaatar Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Species †M. mimicus Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Genus †Sinobaatar Hu & Wang, 2002
- Species †S. lingyuanensis Hu & Wang, 2002
- Species †S. xiei Kusuhashi et al., 2009
- Species †S. fuxinensis Kusuhashi et al., 2009
- Genus †Tedoribaatar Kusuhashi et al., 2008
- Species †T. reini Kusuhashi et al., 2008
- Genus †Teutonodon Martin et al., 2016
- Species †Teutonodon langenbergensis Martin et al. 2016
- Genus †Eobaatar Kielan-Jaworowska, Dashzeveg & Trofimov, 1987
- Family †Albionbaataridae Kielan-Jaworowska & Ensom, 1994
- Genus †Albionbaatar Kielan-Jaworowska & Ensom, 1994
- Species †A. denisae Kielan-Jaworowska & Ensom, 1994
- Genus †Kielanobaatar Kusuhashi et al., 2010
- Species †K. badaohaoensis Kusuhashi et al., 2010
- Genus †Proalbionbaatar Hahn & Hahn, 1998
- Species †P. plagiocyrtus Hahn & Hahn, 1998
- Genus †Albionbaatar Kielan-Jaworowska & Ensom, 1994
- Suborder †Gondwanatheria McKenna 1971 [Gondwanatheroidea Krause & Bonaparte 1993]
- Family †Groeberiidae Patterson, 1952
- Genus †Groeberia Patterson 1952
- Species †G. minoprioi Ryan Patterson, 1952
- Species †G. pattersoni G. G. Simpson, 1970
- Genus †Klohnia Flynn & Wyss 1999
- Species †K. charrieri Flynn & Wyss 1999
- Species †K. major Goin et al., 2010
- Genus ?†Epiklohnia Goin et al., 2010
- Species †Epiklohnia verticalis Goin et al., 2010
- Genus ?†Praedens Goin et al., 2010
- Species †Praedens aberrans Goin et al., 2010
- Genus †Groeberia Patterson 1952
- Family †Ferugliotheriidae Bonaparte, 1986
- Genus †Ferugliotherium Bonaparte, 1986a [Vucetichia Bonaparte, 1990]
- †Ferugliotherium windhauseni Bonaparte, 1986a [Vucetichia gracilis Bonaparte, 1990]
- Genus †Trapalcotherium Rougier et al., 2008
- †Trapalcotherium matuastensis Rougier et al., 2008
- Genus †Ferugliotherium Bonaparte, 1986a [Vucetichia Bonaparte, 1990]
- Family †Sudamericidae Scillato-Yané & Pascual, 1984 [Gondwanatheridae Bonaparte, 1986; Patagonidae Pascual & Carlini, 1987]
- Genus †Greniodon Goin et al., 2012
- †Greniodon sylvanicus Goin et al., 2012
- Genus †Vintana Krause et al., 2014
- †Vintana sertichi Krause et al., 2014
- Genus †Dakshina Wilson, Das Sarama & Anantharaman, 2007
- †Dakshina jederi Wilson, Das Sarama & Anantharaman, 2007
- Genus †Gondwanatherium Bonaparte, 1986
- †Gondwanatherium patagonicum Bonaparte, 1986
- Genus †Sudamerica Scillato-Yané & Pascual, 1984
- †Sudamerica ameghinoi Scillato-Yané & Pascual, 1984
- Genus †Lavanify Krause et al., 1997
- †Lavanify miolaka Krause et al., 1997
- Genus †Bharattherium Prasad et al., 2007
- †Bharattherium bonapartei Prasad et al.,, 2007
- Genus †Patagonia Pascual & Carlini' 1987
- †Patagonia peregrina Pascual & Carlini' 1987
- Genus †Greniodon Goin et al., 2012
- Family †Groeberiidae Patterson, 1952
- Suborder †Cimolodonta McKenna, 1975
- Genus ?†Allocodon non Marsh, 1881
- Species †A. fortis Marsh, 1889
- Species †A. lentus Marsh, 1892 [Cimolomys lentus]
- Species †A. pumilis Marsh, 1892 [Cimolomys pumilus]
- Species †A. rarus Marsh, 1889
- Genus ?†Ameribaatar Eaton & Cifelli, 2001
- Species †A. zofiae Eaton & Cifelli, 2001
- Genus ?†Bubodens Wilson, 1987
- Species †Bubodens magnus Wilson, 1987
- Genus ?†Clemensodon Krause, 1992
- Species †Clemensodon megaloba Krause, 1992 [Kimbetohia cambi, in partim]
- Genus ?†Fractinus Higgins 2003
- Species †Fractinus palmorum Higgins, 2003
- Genus ?†Uzbekbaatar Kielan-Jaworowska & Nesov, 1992
- Species †Uzbekbaatar kizylkumensis Kielan-Jaworowska & Nesov, 1992
- Genus ?†Viridomys Fox 1971
- Species †Viridomys orbatus Fox 1971
- Family †Corriebaataridae Rich et al., 2009
- Genus ?†Corriebaatar Rich et al., 2009
- Species †Corriebaatar marywaltersae Rich et al., 2009
- Genus ?†Corriebaatar Rich et al., 2009
- Paracimexomys group
- Genus Paracimexomys Archibald, 1982
- Species? †P. crossi Cifelli, 1997
- Species? †P. dacicus Grigorescu & Hahn, 1989
- Species? †P. oardaensis (Codrea et al., 2014) [Barbatodon oardaensis Codrea et al., 2014]
- Species †P. magnus (Sahni, 1972) Archibald, 1982 [Cimexomys magnus Sahni, 1972]
- Species †P. magister (Fox, 1971) Archibald, 1982 [Cimexomys magister Fox, 1971]
- Species †P. perplexus Eaton & Cifelli, 2001
- Species †P. robisoni Eaton & Nelson, 1991
- Species †P. priscus (Lillegraven, 1969) Archibald, 1982 [Cimexomys priscus Lillegraven, 1969; genotype Paracimexomys sensu Eaton & Cifelli, 2001]
- Species †P. propriscus Hunter, Heinrich & Weishampel 2010
- Genus Cimexomys Sloan & Van Valen, 1965
- Species †C. antiquus Fox, 1971
- Species †C. gregoryi Eaton, 1993
- Species †C. judithae Sahni, 1972 [Paracimexomys judithae (Sahni, 1972) Archibald, 1982]
- Species †C. arapahoensis Middleton & Dewar, 2004
- Species †C. minor Sloan & Van Valen, 1965
- Species? †C. gratus (Jepson, 1930) Lofgren, 1995 [Cimexomys hausoi Archibald, 1983; Eucosmodon gratus Jepson, 1930; Mesodma ambigua? Jepson, 1940; Stygimus gratus Jepson, 1930]
- Genus †Bryceomys Eaton, 1995
- Species †B. fumosus Eaton, 1995
- Species †B. hadrosus Eaton, 1995
- Species †B. intermedius Eaton & Cifelli, 2001
- Genus †Cedaromys Eaton & Cifelli, 2001
- Genus †Dakotamys Eaton, 1995
- Species? †D. sp. Eaton, 1995
- Species †D. malcolmi Eaton, 1995
- Species †D. shakespeari Eaton 2013
- Genus Paracimexomys Archibald, 1982
- Family †Boffidae Hahn & Hahn, 1983 sensu Kielan-Jaworowska & Hurum 2001
- Genus †Boffius Vianey-Liaud, 1979
- Species †Boffius splendidus Vianey-Liaud, 1979 [Boffiidae Hahn & Hahn, 1983 sensu Kielan-Jaworowska & Hurum, 2001]
- Genus †Boffius Vianey-Liaud, 1979
- Family †Cimolomyidae Marsh, 1889 sensu Kielan-Jaworowska & Hurum, 2001
- Genus †Paressodon Wilson, Dechense & Anderson, 2010
- Species †Paressodon nelsoni Wilson, Dechense & Anderson, 2010
- Genus †Cimolomys Marsh, 1889 [?Allacodon Marsh, 1889; Selenacodon Marsh, 1889]
- Species †C. clarki Sahni, 1972
- Species †C. gracilis Marsh, 1889 [Cimolomys digona Marsh, 1889; Meniscoessus brevis; Ptilodus gracilis Osborn, 1893 non Gidley 1909; Selenacodon brevis Marsh, 1889]
- Species †C. trochuus Lillegraven, 1969
- Species †C. milliensis Eaton, 1993a
- Species ?†C. bellus Marsh, 1889
- Genus ?†Essonodon Simpson, 1927
- Species †E. browni Simpson, 1927 [cimolodontidae? Kielan-Jaworowska & Hurum 2001]
- Genus ?†Buginbaatar Kielan-Jaworowska & Sochava, 1969
- Species †Buginbaatar transaltaiensis Kielan-Jaworowska & Sochava, 1969
- Genus ?†Meniscoessus Cope, 1882 [Dipriodon Marsh, 1889; Tripriodon Marsh, 1889 nomen dubium; Triprotodon Chure & McIntosh, 1989 nomen dubium; Selenacodon Marsh, 1889, Halodon Marsh, 1889, Oracodon Marsh, 1889]
- Species †M. caperatus Marsh, 1889
- Species †M. collomensis Lillegraven, 1987
- Species †M. conquistus Cope 1882
- Species †M. ferox Fox, 1971a
- Species †M. intermedius Fox, 1976b
- Species †M. major (Russell, 1936) [Cimolomys major Russell 1937]
- Species †M. robustus (Marsh, 1889) [Dipriodon robustus Marsh 1889; Dipriodon lacunatus Marsh, 1889; Tripriodon coelatus Marsh, 1889; Meniscoessus coelatus Marsh, 1889; Selenacodon fragilis Marsh, 1889; Meniscoessus fragilis Marsh, 1889; Halodon sculptus (Marsh, 1889); Cimolomys sculptus Marsh, 1889; Meniscoessus sculptus Marsh, 1889; Oracodon anceps Marsh, 1889; Oracodon conulus Marsh, 1892; Meniscoessus borealis Simpson, 1927c; Meniscoessus greeni Wilson, 1987]
- Species †M. seminoensis Eberle & Lillegraven, 1998a
- Genus †Paressodon Wilson, Dechense & Anderson, 2010
- Family †Kogaionidae Rãdulescu & Samson, 1996
- Genus †Kogaionon Rãdulescu & Samson, 1996
- Species †K. ungureanui Rãdulescu & Samson, 1996
- Genus †Hainina Vianey-Liaud, 1979
- Species †H. belgica Vianey-Liaud, 1979
- Species †H. godfriauxi Vianey-Liaud, 1979
- Species †H. pyrenaica Peláez-Campomanes, López-Martínez, Álvarez-Sierra & Daams, 2000
- Species †H. vianeyae Peláez-Campomanes, López-Martínez, Álvarez-Sierra & Daams, 2000
- Genus †Barbatodon Rãdulescu & Samson, 1986
- Species †B. transylvanicum Rãdulescu & Samson, 1986
- Genus †Kogaionon Rãdulescu & Samson, 1996
- Family †Eucosmodontidae Jepsen, 1940 sensu Kielan-Jaworowska & Hurum, 2001 [Eucosmodontidae: Eucosmodontinae Jepsen, 1940 sensu McKenna & Bell, 1997]
- Genus †Eucosmodon Matthew & Granger, 1921
- Species †E. primus Granger & Simpson, 1929
- Species †E. americanus Cope, 1885
- Species †E. molestus Cope, 1869 [Neoplagiaulax molestus Cope, 1869]
- Genus †Stygimys Sloan & Van Valen, 1965
- Species †S. camptorhiza Johnston & Fox, 1984
- Species †S. cupressus Fox, 1981
- Species †S. kuszmauli [Eucosmodon kuszmauli]
- Species †S. jepseni Simpson, 1935
- Species †S. teilhardi Granger & Simpson, 1929
- Genus †Eucosmodon Matthew & Granger, 1921
- Family †Microcosmodontidae Holtzman & Wolberg, 1977 [Eucosmodontidae: Microcosmodontinae Holtzman & Wolberg, 1977 sensu McKenna & Bell, 1997]
- Genus †PentacosmodonJepsen, 1940
- Species †P. pronus Jepsen, 1940 [Djadochtatheroid? (Kielan-Jaworowska & Hurum, 2001)]
- Genus †Acheronodon Archibald, 1982
- Species †A. garbani Archibald, 1982
- Genus †Microcosmodon Jepsen, 1930
- Species †M. conus Jepsen, 1930
- Species †M. rosei Krause, 1980
- Species †M. arcuatus Johnston & Fox, 1984
- Species †M. woodi Holtzman & Wolberg, 1977 [Eucosmodontine?]
- Species †M. harleyi Weil, 1998
- Genus †PentacosmodonJepsen, 1940
- Superfamily †Ptilodontoidea Cope, 1887 sensu McKenna & Bell, 1997 e Kielan-Jaworowska & Hurum, 2001
- Family †Cimolodontidae Marsh, 1889 sensu Kielan-Jaworowska & Hurum, 2001
- Genus †Liotomus Lemoine, 1882 [Neoctenacodon Lemoine 1891]
- Species? †L. marshi (Lemoine, 1882) Cope, 1884 [Neoctenacodon marshi Lemoine, 1882; Neoplagiaulax marshi (Lemoine 1882); Plagiaulax marshi (Lemoine 1882)] [Eucosmodontidae? McKenna & Bell, 1997]
- Genus †Yubaatar Xu et al., 2015
- Species †Yubaatar zhongyuanensis Xu et al., 2015
- Genus †Anconodon Jepsen, 1940
- Species? †A. lewisi (Simpson 1935) Sloan, 1987
- Species †A. gibleyi (Simpson, 1935) [Ptilodus gidleyi Simpson, 1935]
- Species †A. cochranensis (Russell, 1929) [Liotomus russelli (Simpson, 1935); Anconodon russelli (Simpson, 1935) Sloan, 1987; Ectopodon cochranensis (Russell, 1967)]
- Genus †Cimolodon Marsh, 1889 [Nanomys Marsh, 1889, Nanomyops Marsh, 1892]
- Species †C. agilis Marsh, 1889
- Species †C. foxi Eaton, 2002
- Species †C. gracilis Marsh, 1889
- Species †C. electus Fox, 1971
- Species †C. nitidus Marsh, 1889 [Allacodon rarus Marsh, 1892 sensu Clemens, 1964a; Nanomys minutus Marsh, 1889; Nanomyops minutus (Marsh, 1889) Marsh, 1892; Halodon serratus Marsh, 1889; Ptilodus serratus (Marsh, 1889) Gidley 1909]
- Species †C. parvus Marsh, 1889
- Species †C. peregrinus Donohue, Wilson & Breithaupt, 2013
- Species †C. similis Fox, 1971
- Species †C. wardi Eaton, 2006
- Genus †Liotomus Lemoine, 1882 [Neoctenacodon Lemoine 1891]
- Family Incertae sedis
- Genus Neoliotomus Jepsen, 1930
- Species †N. conventus Jepsen, 1930
- Species †N. ultimus (Granger & Simpson, 1928)
- Genus Neoliotomus Jepsen, 1930
- Family †Neoplagiaulacidae Ameghino, 1890 [Ptilodontidae: Neoplagiaulacinae Ameghino, 1890 sensu McKenna & Bell, 1997]
- Genus †Mesodma Jepsen, 1940
- Species? †M. hensleighi Lillegraven, 1969
- Species? †M. senecta Fox, 1971
- Species †M. ambigua Jepsen, 1940
- Species? †M. pygmaea Sloan, 1987
- Species †M. formosa (Marsh, 1889) [Halodon formosus Marsh, 1889]
- Species †M. primaeva (Lambe, 1902)
- Species †M. thompsoni Clemens, 1964
- Genus Ectypodus Matthew & Cranger, 1921 [Charlesmooria Kühne, 1969 ]
- Species †E. aphronorus Sloan, 1981
- Species? †E. childei Kühne, 1969
- Species? †E. elaphus Scott, 2005
- Species? †E. lovei (Sloan, 1966) Krishtlaka & Black, 1975
- Species †E. musculus Matthew & Granger, 1921
- Species †E. powelli Jepsen, 1940
- Species? †E. simpsoni Jepsen, 1930
- Species †E. szalayi Sloan, 1981
- Species †E. tardus Jepsen, 1930
- Genus †Mimetodon Jepsen, 1940
- Species †M. krausei Sloan, 1981
- Species †M. nanophus Holtzman, 1978 [Neoplagiaulax nanophus Holtzman, 1978]
- Species †M. siberlingi(Simpson, 1935) Schiebout, 1974
- Species †M. churchilli Jepsen, 1940
- Genus †Neoplagiaulax Lemoine, 1882
- Species †N. annae Vianey-Liaud, 1986
- Species? †N. burgessi Archibald, 1982
- Species †N. cimolodontoides Scott, 2005
- Species †N. copei Lemoine, 1885
- Species †N. donaldorum Scott & Krause, 2006
- Species †N. eocaenus Lemoine, 1880
- Species †N. grangeri Simpson, 1935
- Species †N. hazeni Jepsen, 1940
- Species †N. hunteri Krishtalka, 1973
- Species †N. jepi Sloan, 1987
- Species †N. kremnus Johnston & Fox, 1984
- Species †N. macintyrei Slaon, 1981
- Species †N. macrotomeus Wilson, 1956
- Species †N. mckennai Sloan, 1987
- Species †N. nelsoni Sloan, 1987
- Species †N. nicolai Vianey-Liaud, 1986
- Species †N. paskapooensis Scott, 2005
- Species? †N. serrator Scott, 2005
- Species †N. sylvani Vianey-Liaud, 1986
- Genus †Parectypodus Jepsen, 1930
- Species †P. armstrongi Johnston & Fox, 1984
- Species? †P. corystes Scott, 2003
- Species? †P. foxi Storer, 1991
- Species †P. laytoni Jepsen, 1940
- Species †P. lunatus Krause, 1982 [P. childei Kühne, 1969]
- Species †P. simpsoni Jepsen, 1940
- Species †P. sinclairi Simpson, 1935
- Species †P. sloani Schiebout, 1974
- Species †P. trovessartianus Cope, 1882 [P. trouessarti; Ptilodus; Mimetodon; Neoplagiaulax]
- Species †P. sylviae Rigsby, 1980 [Ectypodus sylviae Rigby, 1980]
- Species? †P. vanvaleni Sloan, 1981
- Genus †Cernaysia Vianey-Liaud, 1986
- Species †C. manueli Vianey-Liaud, 1986
- Species †C. davidi Vianey-Liaud, 1986
- Genus †Krauseia Vianey-Liaud, 1986
- Species †K. clemensi Sloan, 1981 [Parectypodus clemensi Sloan, 1981]
- Genus †XyronomysRigby, 1980
- Species †X. swainae Rigby, 1980 [Xironomys (sic); ?Eucosmodontidae]
- Genus †Xanclomys Rigby, 1980
- Species †X. mcgrewiRigby, 1980
- Genus †Mesodmops Tong & Wang, 1994
- Species †M. dawsonae Tong & Wang, 1994
- Genus †Mesodma Jepsen, 1940
- Family †Ptilodontidae Cope, 1887 [Ptilodontidae: Ptilodontinae Cope, 1887 sensu McKenna & Bell, 1997]
- Genus †Kimbetohia Simpson, 1936
- Species †K. cambi [Granger, Gregory & Colbert in Matthew, 1937, or Simpson, 1936]
- Species †K. sp. cf. K. cambi
- Genus †Ptilodus Cope, 1881 [Chirox Cope, 1884]
- Species? †P. fractus
- Species †P. kummae Krause, 1977
- Species †P. gnomus Scott, Fox & Youzwyshyn, 2002 [cf. Ectypodus hazeni (Jepsen, 1940) Gazin, 1956]
- Species †P. mediaevus Cope, 1881 [Ptilodus plicatus (Cope, 1884); Chirox plicatus Cope, 1884 P. ferronensis Gazin, 1941]
- Species †P. montanus Douglass, 1908 [P. gracilis Gidley, 1909; P. admiralis Hay, 1930]
- Species †P. tsosiensis Sloan, 1981
- Species †P. wyomingensis Jepsen, 1940
- Genus †Baiotomeus Krause, 1987
- Species †B. douglassi Simpson, 1935 [Ptilodus; Mimetodon; Neoplagiaulax]
- Species †B. lamberti Krause, 1987
- Species †B. russelli Scott, Fox & Youzwyshyn, 2002
- Species †B. rhothonion Scott, 2003
- Genus †Prochetodon Jepsen, 1940
- Genus †Kimbetohia Simpson, 1936
- Family †Cimolodontidae Marsh, 1889 sensu Kielan-Jaworowska & Hurum, 2001
- Superfamily †Taeniolabidoidea Granger & Simpson, 1929 sensu Kielan-Jaworowska & Hurum, 2001
- Genus †Prionessus Matthew & Granger, 1925
- Species †P. lucifer Matthew & Granger, 1925
- Family †Lambdopsalidae
- Genus †Lambdopsalis Chow & Qi, 1978
- Species †L. bulla Chow & Qi, 1978
- Genus †Sphenopsalis Matthew, Granger & Simpson, 1928
- Species †S. nobilis Matthew, Granger & Simpson, 1928
- Genus †Lambdopsalis Chow & Qi, 1978
- Family †Taeniolabididae Granger & Simpson, 1929
- Genus †Taeniolabis Cope, 1882
- Species †T. lamberti Simmons, 1987
- Species †T. taoensis Cope, 1882
- Genus †Kimbetopsalis
- Species †K. simmonsae
- Genus †Taeniolabis Cope, 1882
- Genus †Prionessus Matthew & Granger, 1925
- Superfamily †Djadochtatherioidea Kielan-Jaworowska & Hurum, 1997 sensu Kielan-Jaworowska & Hurum, 2001[Djadochtatheria Kielan-Jaworowska & Hurum, 1997]
- Genus? †Bulganbaatar Kielan-Jaworowska, 1974
- Species? †B. nemegtbaataroides Kielan-Jaworowska, 1974
- Genus †Nemegtbaatar Kielan-Jaworowska, 1974
- Species? †N. gobiensis Kielan-Jaworowska, 1974
- Family †Chulsanbaataridae Kielan-Jaworowska, 1974
- Genus †Chulsanbaatar Kielan-Jaworowska, 1974
- Species †C. vulgaris Kielan-Jaworowska, 1974
- Genus †Chulsanbaatar Kielan-Jaworowska, 1974
- Family †Sloanbaataridae Kielan-Jaworowska, 1974
- Genus †Kamptobaatar Kielan-Jaworowska, 1970
- Species? †K. kuczynskii Kielan-Jaworowska, 1970
- Genus †Nessovbaatar Kielan-Jaworowska & Hurum, 1997
- Species †N. multicostatus Kielan-Jaworowska & Hurum, 1997
- Genus †Sloanbaatar Kielan-Jaworowska, 1974
- Species †S. mirabilis Kielan-Jaworowska, 1974 [Sloanbaatarinae]
- Genus †Kamptobaatar Kielan-Jaworowska, 1970
- Family †Djadochtatheriidae Kielan-Jaworowska $ Hurum, 1997
- Genus †Djadochtatherium Simpson, 1925
- Species †D. matthewi Simpson, 1925[Catopsalis matthewi Simpson, 1925]
- Genus †Catopsbaatar Kielan-Jaworowska, 1974
- Species †C. catopsaloides (Kielan-Jaworowska, 1974) Kielan-Jaworowska, 1994 [Djadochtatherium catopsaloides Kielan-Jaworowska, 1974]
- Genus †Tombaatar Kielan-Jaworowska, 1974
- Species †T. sabuli Rougier, Novacek & Dashzeveg, 1997
- Genus †Kryptobaatar Kielan-Jaworowska, 1970 [Gobibaatar Kielan-Jaworowska, 1970, Tugrigbaatar Kielan-Jaworowska & Dashzeveg, 1978]
- Species †K. saichanensis Kielan-Jaworowska & Dashzeveg, 1978 [Tugrigbaatar saichaenensis Kielan-Jaworowska & Dashzeveg, 1978??]
- Species †K. dashzevegi Kielan-Jaworowska, 1970
- Species †K. mandahuensis Smith, Guo & Sun, 2001
- Species †K. gobiensis Kielan-Jaworowska, 1970 [Gobibaatar parvus Kielan-Jaworowska, 1970 ]
- Genus †Djadochtatherium Simpson, 1925
- Genus? †Bulganbaatar Kielan-Jaworowska, 1974
- Genus ?†Allocodon non Marsh, 1881
Paleoecology
Evolutionary history
The multituberculates existed for about 166 or 183 million years, and are often considered the most successful, diversified, and long-lasting mammals in natural history.[8] They first appeared in the Jurassic, or perhaps even the Triassic, survived the mass extinction in the Cretaceous, and became extinct in the early Oligocene epoch, some 35 million years ago.[8] The oldest known species in the group is Indobaatar zofiae from the Jurassic of India, some 183 million years ago,[23] and the youngest are two species, Ectypodus lovei and an unnamed possible neoplagiaulacid, from the late Eocene/Oligocene Medicine Pole Hills deposits of North Dakota.[24] If gondwanatheres are multituberculates, then the clade might have survived even longer into the Colhuehuapian Miocene in South America, in the form of Patagonia peregrina.[2]
Behaviour
Multituberculates are some of the earliest mammals to display complex social behaviours.[25]
Extinction
The extinction of multituberculates has been a topic of controversy for several decades.[26] After at least 88 million years of dominance over most mammalian assemblies, multituberculates reached the peak of their diversity in the early Palaeocene, before gradually declining across the final stages of the epoch and the Eocene, finally disappearing in the early Oligocene (mid-Miocene if gondwanatherians are multituberculates).[27] Traditionally, the extinction of multituberculates has been linked to the rise of rodents (and, to a lesser degree, earlier placental competitors like hyopsodonts and Plesiadapiformes), which supposedly competitively excluded multituberculates from most mammalian faunas.[5]
However, the idea that multituberculates were replaced by rodents and other placentals has been criticised by several authors. For one thing, it relies on the assumption that these mammals are "inferior" to more derived placentals, and ignores the fact that rodents and multituberculates had co-existed for at least 15 million years. According to some researchers, multituberculate "decline" is shaped by sharp extinction events, most notably after the Tiffanian, where a sudden drop in diversity occurs. Finally, the youngest known multituberculates do not exemplify patterns of competitive exclusion; the Oligocene Ectypodus is a rather generalistic species, rather than a specialist. This combination of factors suggests that, rather than gradually declining due to pressure from rodents and similar placentals, multituberculates simply could not cope with climatic and vegetation changes, as well as the rise of new predatory eutherians, such as miacids.[27]
More recent studies show a mixed effect. Multituberculate faunas in North America and Europe do indeed decline in correlation to the introduction of rodents in these areas. However, Asian multituberculate faunas co-existed with rodents with minimal extinction events, implying that competition was not the main cause for the extinction of Asiatic multituberculates. As a whole, it seems that Asian multituberculates, unlike North American and European species, never recovered from the KT event, which allowed the evolution and propagation of rodents in the first place.[26] A recent study seems to indeed indicate that eutherians recovered more quickly from the KT event than multituberculates.[28]
Competition between gondwanatherians and rodents and/or other Glires is untested, with a wide span of time between the youngest representatives of the former in India, Africa and Madagascar in the Maastrichtian and the first representatives of the latter in the Palaeocene,[29] Eocene[30] and Oligocene[31] respectively. Co-existence between both groups is currently confirmed only in South America, Patagonia peregrina is thought to have been forced into a specialised fossorial niche by competition with rodents and argyrolagoidean paucituberculate marsupials,[2] but another clade, Groeberiidae, attained its peak diversity in the mid-Oligocene, after the arrival of rodents.[32]
The Saint Bathans mammal, a small mammal fossil known from New Zealand's Miocene (Manuherikia Group, dated to 19 million years ago), may have been a multituberculate. If so, it is the most recent known multituberculate to exist. It is probably a relict species that was on New Zealand when it split off from Gondwana 80 million years ago.
Geographic distribution
Multituberculates are mostly known from the northern continents (Laurasia), but there are various records from the southern continents (Gondwana). The group Gondwanatheria, known from Argentina, Antarctica, Madagascar, India, and possibly Tanzania, has been referred to the order in the past and, while this placement remains controversial, most recent phylogenetic studies recover them as multituberculates outside but close to Cimolodonta.[1][33][34][2] Two genera, Hahnodon and Denisodon, are known from the Early Cretaceous of Morocco, but they may instead be haramiyidans.[35][36] Multituberculates have also been recorded from the Late Cretaceous of Madagascar and Argentina.[37][38] An Australian multituberculate, Corriebaatar, is known from a single tooth.[39] Indobaatar is known from the Kota Formation of India - then part of eastern Gondwanna - and is the oldest known multituberculate.[23]
In the late Cretaceous, multituberculates were widespread and diverse in the northern hemisphere, and possibly across most southern landsmasses as well, making up more than half of the mammal species of typical faunas. Although several lineages became extinct during the faunal turnover at the end of the Cretaceous, multituberculates as a whole managed very successfully to cross the Cretaceous–Paleogene boundary and reached their peak of diversity during the Paleocene. They were an important component of nearly all Paleocene faunas of Europe and North America, and of some late Paleocene faunas of Asia. Multituberculates were also most diverse in size during the Paleocene, ranging from the size of a very small mouse to that of a panda. However, in Asia, Palaeocene and Eocene multituberculates compose a very small percentage of the overall local mammalian fauna, having never managed to recover from the KT event in the same way that their North American and European counterparts did.[26] Gondwanatheres are common in the Late Cretaceous of Madagascar and India, the Paleocene and Eocene of Seymour Island, and occur in South America from the Late Cretaceous to the Miocene.
References
- Krause, David W.; Hoffmann, Simone; Wible, John R.; Kirk, E. Christopher; Schultz, Julia A.; von Koenigswald, Wighart; Groenke, Joseph R.; Rossie, James B.; O’Connor, Patrick M.; Seiffert, Erik R.; Dumont, Elizabeth R.; Holloway, Waymon L.; Rogers, Raymond R.; Rahantarisoa, Lydia J.; Kemp, Addison D.; Andriamialison, Haingoson (November 2014). "First cranial remains of a gondwanatherian mammal reveal remarkable mosaicism". Nature. 515 (7528): 512–517. Bibcode:2014Natur.515..512K. doi:10.1038/nature13922. PMID 25383528. S2CID 4395258.
- Nicolás R. Chimento, Federico L. Agnolin and Fernando E. Novas (2015). "The bizarre 'metatherians' Groeberia and Patagonia, late surviving members of gondwanatherian mammals". Historical Biology: An International Journal of Paleobiology. 27 (5): 603–623. doi:10.1080/08912963.2014.903945. S2CID 216591096.CS1 maint: uses authors parameter (link)
- Brandt, Riley (July 6, 2018). "56-million-year-old fossils complicate long-held theories about mammalian body size". phys.org. Retrieved 8 July 2018.
- Swales, Jude (March 14, 2012). "Some mammals used highly complex teeth to compete with dinosaurs: study". phys.org. doi:10.1038/nature10880. Retrieved 8 July 2018.
- Krause, David W. (1986). Competitive exclusion and taxonomic displacement in the fossil record; the case of rodents and multituberculates in North America. Rocky Mountain Geology. 24. pp. 95–117. doi:10.2113/gsrocky.24.special_paper_3.95. ISBN 978-0941570022.
- Weil, Anne (June 1997). "Introduction to Multituberculates: The 'Lost Tribe' of Mammals". Berkeley: UCMP.
- Chen, Meng; Philip Wilson, Gregory (2015). "A multivariate approach to infer locomotor modes in Mesozoic mammals". Paleobiology. 41 (2): 280–312. doi:10.1017/pab.2014.14. S2CID 86087687.
- Agustí-Antón 2002, pp 3-4
- Benton, Michael J. Vertebrate Palaeontology (2004), p. 300
- Carrano, Matthew T., and Richard W. Blob, Timothy J. Gaudin, and John R. Wible (2006). Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles, p. 358.
- Celik, Mélina A.; Phillips, Matthew J. (8 July 2020). "Conflict Resolution for Mesozoic Mammals: Reconciling Phylogenetic Incongruence Among Anatomical Regions". Frontiers in Genetics. 11: 0651. doi:10.3389/fgene.2020.00651. PMC 7381353. PMID 32774343.
- Kielan-Jaworowska, Zofia, Richard L. Cifelli, and Zhe-Xi Luo (2005). Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure , p. 299
- Gurovich 2005 p. 334; Gurovich & Beck 2009, pp. 31–32; Rougier et Al. 2009 p.233
- Lanyon, J. M.; Sanson, G. D. (February 2006). "Degenerate dentition of the dugong (Dugong dugon), or why a grazer does not need teeth: morphology, occlusion and wear of mouthparts". Journal of Zoology. 268 (2): 133–152. doi:10.1111/j.1469-7998.2005.00004.x.
- Williamson, Thomas E.; Brusatte, Stephen L.; Secord, Ross; Shelley, Sarah (2015). "A new taeniolabidoid multituberculate (Mammalia) from the middle Puercan of the Nacimiento Formation, New Mexico, and a revision of taeniolabidoid systematics and phylogeny". Zoological Journal of the Linnean Society. 177: 183–208. doi:10.1111/zoj.12336.
- Gondwanatheria
- "Article expired". 2013-05-10.
- Crompton, A.W.; Musinsky, C.; Rougier, G.W.; Bhullar, B.-A.S.; Miyamae, J. A. (2018). "Origin of the Lateral Wall of the Mammalian Skull: Fossils, Monotremes and Therians Revisited". Journal of Mammalian Evolution. 25 (3): 301–313. doi:10.1007/s10914-017-9388-7. S2CID 16072755.
- Longrich, Nicholas R.; Ryan, Michael J. (2010). "Mammalian tooth marks on the bones of dinosaurs and other Late Cretaceous vertebrates". Palaeontology. 53 (4): 703–709. doi:10.1111/j.1475-4983.2010.00957.x.
- Williamson, Thomas E.; Brusatte, Stephen L.; Secord, Ross; Shelley, Sarah (2015). "A new taeniolabidoid multituberculate (Mammalia) from the middle Puercan of the Nacimiento Formation, New Mexico, and a revision of taeniolabidoid systematics and phylogeny". Zoological Journal of the Linnean Society. 177: 183–208. doi:10.1111/zoj.12336.
Taeniolabidoids underwent a modest taxonomic radiation during the early Palaeocene of North America and underwent a dramatic increase in body size, with Taeniolabis taoensis possibly exceeding 100 kg
- Dykes Multituberculata (Cope 1884)
- Mikko's Phylogeny Archive Haaramo, Mikko (2007). "Mammaliaformes – mammals and near-mammals". Retrieved 30 December 2015.
- Parmar, Varun; Prasad, Guntupalli V. R.; Kumar, Deepak (June 2013). "The first multituberculate mammal from India". Naturwissenschaften. 100 (6): 515–523. Bibcode:2013NW....100..515P. doi:10.1007/s00114-013-1047-0. PMID 23644519. S2CID 18371712.
- Schumaker, Karew; Kihm, Allen J. (2006). "Multituberculates from the Medicine Pole Hills Local Fauna (Chadronian) of Bowman, County, North Dakota" (PDF). Paludicola. 6 (1): 9–21. S2CID 91179140.
- Weaver, Lucas N.; Varricchio, David J.; Sargis, Eric J.; Chen, Meng; Freimuth, William J.; Wilson Mantilla, Gregory P. (2 November 2020). "Early mammalian social behaviour revealed by multituberculates from a dinosaur nesting site". Nature Ecology & Evolution: 1–6. doi:10.1038/s41559-020-01325-8. PMID 33139921.
- Wood, D. Joseph (2010). The Extinction of the Multituberculates Outside North America: a Global Approach to Testing the Competition Model (M.S.). The Ohio State University. Archived from the original on 2015-04-08. Retrieved 2015-04-03.
- Ostrander, Gregg E. (1984). "The Early Oligocene (Chadronian) Raben Ranch Local Fauna, Northwest Nebraska: Multituberculata; with Comments on the Extinction of the Allotheria". Transactions of the Nebraska Academy of Sciences. 10: 71–80.
- Pires, Mathias M.; Rankin, Brian D.; Silvestro, Daniele; Quental, Tiago B. (1804). "Diversification dynamics of mammalian clades during the K–Pg mass extinction". Biology Letters. 14 (9): 2058. doi:10.1098/rsbl.2018.0458. PMC 6170748. PMID 30258031.
- Rose, K.D.; Deleon, V.B.; Mmissian, P.; Rana, R.S.; Sahni, A.; Singh, L.; Smith, T. (2007). "Early Eocene lagomorph (Mammalia) from western India and the early diversification of Lagomorpha". Proceedings of the Royal Society B. 275 (1639): 1203–1208. doi:10.1098/rspb.2007.1661. PMC 2602686. PMID 18285282.
- Marivaux, Laurent; Essid, El Mabrouk; Marzougui, Wissem; Ammar, Hayet Khayati; Adnet, Sylvain; Marandat, Bernard; Merzeraud, Gilles; Tabuce, Rodolphe; Vianey-Liaud, Monique (2014). "A new and primitive species of Protophiomys (Rodentia, Hystricognathi) from the late middle Eocene of Djebel el Kébar, Central Tunisia" (PDF). Palaeovertebrata. 38 (1): 1–17. doi:10.18563/pv.38.1.e2.
- Poux, C.; Madsen, O.; Marquard, E.; Vieites, D. R.; de Jong, W. W.; Vences, M. (2005). "Asynchronous colonization of Madagascar by the four endemic clades of primates, tenrecs, carnivores, and rodents as inferred from nuclear genes". Systematic Biology. 54 (5): 719–730. doi:10.1080/10635150500234534. PMID 16243759.
- Goin, F.J., Abello M.A. & Chornogubsky L. 2010. Middle Tertiary marsupials from Central Patagonia (Early Oligocene of Gran Barranca): Understanding South America's Grande Coupure. En: Madden R.H., Carlini A.A., Vucetich M.G. & Kay R.F. (Eds.), The Paleontology of Gran Barranca: Evolution and Environmental Change through the Middle Cenozoic of Patagonia. Cambridge University Press.
- Drake, Nadia (November 5, 2014). "Fossil From Dinosaur Era Reveals Big Mammal With Super Senses". nationalgeographic.com. National Geographic Society. Retrieved November 5, 2014.
- Wilford, John Noble (November 5, 2014). "Fossil's Unusual Size and Location Offer Clues in Evolution of Mammals". New York Times. Retrieved November 6, 2014.
- Anantharaman, S.; Wilson, G. P.; Sarma, D. C. Das; Clemens, W. A. (12 June 2006). "A possible Late Cretaceous 'Haramiyidan' from India". Journal of Vertebrate Paleontology. 26 (2): 488–490. doi:10.1671/0272-4634(2006)26[488:APLCHF]2.0.CO;2.
- Ashok Sahni, R. S. Rana and G. V. R. Prasad (1987). "New evidence for palaeogeographic intercontinental Gondwana relationships based on Late Cretaceous-Earliest Palaeocene coastal faunas from peninsular India". In McKenzie, Garry D. (ed.). Gondwana Six: Stratigraphy, Sedimentology, and Paleontology. Geophysical Monograph Series. American Geophysical Union. pp. 207–218. doi:10.1029/GM041p0207. ISBN 978-0-87590-067-4.CS1 maint: uses authors parameter (link)
- Gurovich, Y.; Beck, R. (2009). "The phylogenetic affinities of the enigmatic mammalian clade Gondwanatheria". Journal of Mammalian Evolution. 16 (1): 25–49. doi:10.1007/s10914-008-9097-3. S2CID 42799370.
- Krause, David W.; Hoffmann, Simone; Werning, Sarah (2017). "First postcranial remains of Multituberculata (Allotheria, Mammalia) from Gondwana". Cretaceous Research. 80: 91–100. doi:10.1016/j.cretres.2017.08.009.
- Rich, T. H.; Vickers-Rich, P.; Flannery, T. F.; Kear, B. P.; Cantrill, D. J.; Komarower, P.; Kool, L.; Pickering, D.; Rusler, P.; Morton, S.; van Klaveren, N.; Fitzgerald, E. M. G. (2009). "An Australian multituberculate and its palaeobiogeographic implications". Acta Palaeontologica Polonica. 54 (1): 1–6. CiteSeerX 10.1.1.527.9540. doi:10.4202/app.2009.0101. S2CID 30933794.
Sources
| Wikimedia Commons has media related to Multituberculata. |
| Wikispecies has information related to Multituberculata. |
- Agustí, Jordi; Antón, Mauricio (2002). Mammoths, Sabertooths, and Hominids: 65 Millions Years of Mammalian Evolution in Europe. New York: Columbia University Press. ISBN 978-0-231-11640-4.
- Dykes, Trevor. "Multituberculata (Cope 1884)". Archived from the original on December 28, 2009.
- Kielan-Jaworowska, Zofia; Hurum, Jørn H. (2001). "Phylogeny and Systematics of multituberculate mammals". Palaeontology. 44 (3): 389–429. doi:10.1111/1475-4983.00185.

