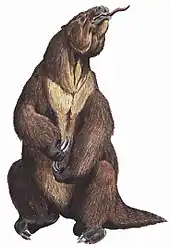
Mylodon
Mylodon is a genus of extinct ground sloth belonging to the family Mylodontidae, known from the region of Patagonia in Chile and Argentina in southern South America. With a total length of 3 to 4 m, it is one of the best-known and largest representatives of the group. The oldest finds probably date to the Lower Pleistocene. Most of the fossil remains, however, date from the Upper Pleistocene period. One of the most important sites of this phase is the Cueva del Milodón in southern Chile. Shortly after, about 10,200 BP,[1] Mylodon became extinct. At this point in time, it coexisted with the first human colonists in America. However, there is little evidence that it was hunted by humans.
| Mylodon | |
|---|---|
.jpg.webp) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Pilosa |
| Family: | †Mylodontidae |
| Genus: | †Mylodon Owen, 1840 |
| Species: | †M. darwini |
| Binomial name | |
| †Mylodon darwini Owen, 1840 | |
In Mylodon's case, not only bones and teeth are known, but also various soft tissue and integumentary structures are preserved. The diet of Mylodon is known in great detail due to fossilized faeces. Its skull is greatly elongated and, compared to other large mylodontids, is narrower, possessing a completely closed nasal arch. Other distinguishing features concern the dental structure.
Mylodon was a terrestrial ground sloth. A thick coat with long hair can be interpreted as an adaptation to a life under cold climatic conditions, as they prevailed in southern South America during the last cold period. A diet based predominantly on grasses also corresponds to this in this region. The widespread distribution of Mylodon into the pampas region and some features on the skull show, however, that the animals had a much larger ecological range and could also cope with warmer temperature conditions and possibly a mixed vegetable diet. Some of the animals fell victim to larger predators .
The genus was described in 1840, usually only one species is recognized. The type material comes from the area of the Pampas, where it was collected by Charles Darwin during his voyage with the HMS Beagle. Mylodon was one of the first extinct sloths on which genetic studies were carried out.
Discovery

Mylodon was named by Richard Owen on the basis of a nearly complete lower jaw with teeth, which was found by Charles Darwin in a consolidated gravel cliff at Bahía Blanca, during the survey expedition of HMS Beagle.[2] At several sites preserved hide and dung have been discovered, and are in such a state of conservation that the people who first discovered them believed they belonged to a living animal instead of an extinct species. The discovery of fresh looking samples of skin and dung sparked a small wave of expeditions during the early 20th century to search for a living example of the animal.[3] The samples have since been found to be around 10,000 years old, although they look fresh because of the extreme cold and stable conditions in the caves in which they were found.
Fossils assigned to Mylodon have also been found in the Ñuapua Formation of Bolivia.[4] Well preserved samples of Mylodon remains have been discovered in the Cueva del Milodón site in Patagonia, Chile along the southern flank of Cerro Benítez in the year 1896. Associated with bones of other early Patagonian animals, these remains of Mylodon date from an era earlier than 10,000 BC.[5] The American Museum of Natural History has exhibited a sample of Mylodon dung from Argentina with a note that reads "deposited by Theodore Roosevelt".[6][7][8][9]
Description
General
Mylodon was a large representative of the Mylodontidae. Its total length was estimated to be around 3 to 4 m. Based on the size of the skull, a weight between 1 and 2 tonnes is assumed, with an approximate estimate of 1.65 tonnes.[10] Thus, Mylodon had about the size of related forms such as Glossotherium or Paramylodon, but was significantly smaller than the giant Lestodon. In terms of physique, it largely corresponded with the other large ground-living sloths.[6][11]
Skull and dentition features
Especially in the construction of the skull, Mylodon differed significantly from other related forms. Its length varied between 59 and 71.5 cm, which is significantly longer than Glossotherium or Lestodon. At the skull it was between 16.5 and 22.5 cm wide, in the front nasal area between 11.3 and 15.5 cm. The height of the posterior skull was 14.0 to 19.0 cm and the anterior 15.0 to 23.5 cm.[12] The skull was thereby elongated and narrow, unlike Glossotherium and Lestodon that had a short and very broad skull. The extraordinary length of the skull of Mylodon was mainly due to elongations in the rostrum. Seen from above, the rostrum narrowed towards the front. This is where the most important difference to most of the other representatives of the Mylodontidae can be found: The nasal bone was long and narrow and curved downwards in the front area. At the front end, it connected to the middle jawbone, which was lengthened by an appendage, and which in turn fused with the upper jaw. This resulted in a completely closed nasal arch in adult individuals, which is largely unknown in other sloths. In comparison, the skulls of Glossotherium and Lestodon, but also of Paramylodon, showed a nasal area, seen from above, which was rather short and looked clearly cut off when viewed from the side; The roof of the skull was largely straight in Mylodon, only a slight indentation could occur above the orbit. On parietal, significant temporal lines were present, but no head crest formed. The zygomatic arch was slim, the anterior attachment began above the third and fourth molars. It did not form a solid end with the rear arch attachment. As is usual with sloths, the front arch base consisted of three appendages: one ascending, one horizontal, and one descending, the former of which was the longest. The rear arch formed a triangular plate. The occiput bent at an angle of 120 ° from the roof of the skull. The underside of the occiput was at about the level of the occlusal plane. When viewed from behind, the occiput appeared almost circular and not as depressed as in Glossotherium and Lestodon. [13] The palate was narrow and was more or less triangularly oriented towards the front of the skull. Numerous small bone openings were characteristic here. The glenoid pit, in which the joint of the lower jaw engages, corresponding to that of other mylodonts with its weak form, but this provided free rotation overall.[12][14][15]

The lower jaw of Mylodon varied in length between 42 and 48 cm. It was elongated, more noticeable than in Glossotherium and Lestodon, since in Mylodon the area in front of the teeth, in particular, had stretched. The horizontal bone body increased continuously in height towards the rear, below the last molar it was about 10.5 to 12.7 cm. The symphysis at the front end for the jointing of the two halves of the lower jaw was about 12.4 cm long. Here the lower edge of the body of the lower jaw rose at an angle so that the anterior end of the symphysis was above the occlusal plane of the teeth. As with other sloths, the symphysis extended forward, it ended slightly rounded. According to the rostrum of the skull, Mylodon's symphysis was narrow and not as wide as in Glossotherium and Lestodon. The mandible foramen opened shortly behind the symphysis. The ascending branch started behind the last molar and formed an angle of 140 ° to the occlusal plane. The crown process rose up to 20 cm. In contrast, the articular process was lower, roughly at the level of the occlusal plane, resulting in a low cranial-mandibular connection. The angular process at the rear end of the lower jaw was clearly visible. Sometimes it tipped down and was below the lower edge of the horizontal bone body. The upper side of the angular process does not reach the occlusal plane.[12][14][15]
The dentition of Mylodon differs greatly from that of the other placental mammals and usually consists of five teeth at the top and four teeth at the bottom per jaw arch, meaning a total of 18 teeth. In the mylodonts, the first tooth was often caniniform while the rear teeth were more molariform. Within the sloth, this structure of the teeth can be called original. A special feature of Mylodon was that the upper canine-like tooth of each row was completely regressed and only the molar-like four rear teeth were found here. In the lower row of teeth, the anterior caniniform tooth was transformed into a molariform. The dentition thus consisted of a total of 16 teeth. This is somewhat reminiscent of Paramylodon, in which the upper canine-shaped teeth were also missing, but the lower ones had retained their strikingly pointed shape. In contrast to this, Glossotherium and Lestodon had the original decayed teeth. The flat, flap-like and largely indented structure of the molariform teeth can be emphasized as a characteristic of the mylodonts, which clearly differs from that of the Megatheriidae and Megalonychidae with their two transverse raised ridges per tooth. The shapes of the teeth present in Mylodon were simpler. In the upper jaw row, they had a rather round to oval outline, in the lower jaw row a more diamond-shaped outline. The typically more complex bilobed design of the molar-like teeth of Glossotherium and Lestodon , caused by a central constriction, only occurred on the lower rearmost tooth in Mylodon. In general, the rows of teeth diverged to the front, and the teeth were very high crowned (hypsodont). The upper row of teeth stretched from 10.9 to 13.3 cm, the lower row was between 12.0 and 15.0 cm.[14][12][15][16]
Postcrania
Postcranial skeletons are far rarer in Mylodon than in the other large mylodontid sloths. As a result, the skeleton is less well documented. Only individual elements of the spine, such as the atlas and various thoracic vertebrae, have been described. The humerus was massive and extremely long at 46 to 48 cm. The joint head, the diameter of which was over 10 cm, stood out due to its hemispherical, but laterally somewhat flattened shape. A distinct deltopectoral ridge ran down the shaft, which acted as an anchor point for the shoulder muscles. As with many ground sloths, the lower end of the joint extended far and brought it here to a width of almost 26 cm. In part, this was caused by a massive internal epicondyle. The articular surfaces (capitulum and trochlea) were almost perpendicular to each other and did not form such an obtuse angle as in Glossotherium. The cubit was built gracefully. Their length was around 37 cm. The olecranon, i.e. the upper articular process, took up about 8.1 cm of it, which corresponds to about 22% of the total length and is significantly less than in comparison with Glossotherium and Lestodon. It was laterally narrowed, which is also found in Paramylodon. The spoke largely resembled that of Glossotherium and was compact and straight built with a length of about 30 cm. The head was oval in shape with a prominent lip. The pelvis was extremely expansive and 114 cm wide between the two iliac bones. The thigh bone measured between 55 and 59 cm in length. It was typical of ground sloths, being flat in shape. Its width decreased significantly on the shaft, the lowest value was reached just below the midpoint. Here the width was about 18 cm, the thickness about 7.5 cm. The joint ends, on the other hand, were markedly wider, around 30 cm at the knee end and around 26 cm at the foot end. The hemispherical joint head with a diameter of a good 14 cm towered over the Great Rolling Hill. The third rolling mound was only visible as a small elevation on the outer edge of the shaft below the large rolling mound. Opposite the thighbone reached the shin with about 27 cm only about half the length, a characteristic of Mylodonts. This bone, too, was clearly flat with a thickness that was only half the value of the width at the shaft. The fibula is so far only fragmented. It was drawn in on the shaft and widened at the joint ends, with the upper joint end showing more pronounced curves than in Glossotherium./[17][18][19]
The hand comprised a total of five digits (I to V), whereby the metacarpal bone was fused with the large polygonal bone on the first digit. This created the so-called Metacrapal Carpal Complex (MCC for short), which is typical for many ground sloths. As a special feature of the wrist, the pea bone was clearly flat, its shape resembled that of Glossotherium, but differed from the corresponding bone of other Mylodonts with spherical, walnut-like or a pyramidal shape. The fourth digit had formed the longest metacarpal bone, while that of the fifth was only slightly shorter. The respective bones measured there around 12.5 and 10.7 cm in length. As with Glossotherium and Paramylodon, only the three inner digit were probably clawed, but only of the second digit have all bone elements been documented. The metacarpal bone was 7.8 cm long and was built very gracefully. The first phalanx was extremely short and only about 2.5 cm long, the second was about 4.2 cm long and the third at least 11.5 cm. It was tubular and went forward into an extension on which the claw rested. The first phalanges of the two outer digits were significantly reduced in length. Only individual root bones of the foot, such as the talus, are present.[18]
Integument

Mylodon is one of the few extinct mammals that has mummified skin remains. The most important location for such finds is the Cueva del Milodón in the Chilean province of Última Esperanza, where the first skin parts were brought to light at the end of the 19th century.[20] [21] Individual pieces have lengths of up to 150 cm, but have shrunk through drying processes. Its thickness is up to 1.5 cm in some places, but it is usually around 1 cm. The skin is densely covered with stiff, slightly wavy hair, with only the top hair being developed, while the undercoat is missing. This feature is similar to the two-toed sloths but less so than the three-toed sloths), which possess an undercoat. The length of the individual hairs vary between 5 and sometimes over 20 cm with the shortest in the area of the back of the head, medium-length hair on the back and very long hair on the limbs. Their known color ranges from yellowish to reddish-brown. The hair shafts are uniformly tubular, at the upper end they form blunt tips. As with today's sloths, the hair did not have a pith (medulla). In contrast to the hair of the two-toed sloth, they lack their characteristic longitudinal ribbing.[20][21][22][23][24]
The mylodonts are the only representatives of the sloths to have bony plates embedded in their skin. Such structures, called osteoderms, are known today to a greater extent only in armadillos. In contrast to the outer armor of the armadillos, the bone platelets of the Mylodonts were rather loosely scattered. Hermann Burmeister published the first finds of individual osteoderms of Mylodon as early as the 1860s.[25][26] The remains of skin found in the caves of Última Esperanza give an impression of how they were embedded in the skin and distributed over the body. The bone platelets are all located in the lower section of the skin, while the hairs originate in the upper sections. The distribution turned out to be very inconsistent. Some areas with a dense array of osteoderms contain between 83 and 95 platelets per 10 cm². For others, however, the number is very thin. However, even with a close arrangement, the osteoderms never unite to form a closed shell, but are always separated from one another by individual skin folds. In accordance with the armadillos' shells, the bone platelets form a single layer and do not appear stacked. Since all skin residues were found isolated from the body skeletons, it is sometimes difficult to assign the skin areas with a dense and thin arrangement of bone platelets to a specific part of the body. However, it can be assumed that the back was largely armored and the stomach was free. In the sections with dense osteoderm formation, these were larger than in the clear areas. The bone platelets of Mylodon were mostly of irregular oval shape with dimensions of 0.5 to 2.5 cm in length, 0.3 to 1.8 cm in width and 0.2 to 1.1 cm in thickness, with weights of a maximum of 2g. On the surface, they showed individual dimples.[27] In cross-section, they consisted of numerous bundles of fibers mixed with hard bone blades ( osteoma ). This made their structure much simpler than that of the armadillos, and they probably lacked the keratin layer known from the armadillos. In principle, the osteoderms of Mylodon were similar to those of other large mylodonts.[20][21][28][29][27][30]
Taxonomy
Mylodon's close relatives include the ground sloths of the genera Glossotherium and Paramylodon. The latter genus has often been confused with Glossotherium, but Paramylodon is a distinct genus that was restricted to the Pleistocene of North America.[15] Glossotherium also shares a long history of taxonomic confusion with Mylodon, and currently the only recognized species is Mylodon darwini. At one time, the elephant-sized Megatherium was thought to be closely related, but is recognized as belonging to a separate family (Megatheriidae).
Below is a phylogenetic tree of the Mylodontidae, based on Boscaini et al 2019.[31]
| Mylodontidae |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Recent molecular sequence results obtained using collagen[32] and mitochondrial DNA[33] extracted from fossils indicate that the closest living relatives of Mylodon are the two-toed sloths of genus Choloepus. This revelation came as a surprise, since morphological analyses had previously suggested that two-toed sloths were close to Caribbean sloths and Megalonyx, now regarded as representing two separate and distant branches of the sloth evolutionary tree.
Paleobiology

The mylodontids (particularly Mylodon itself) are often considered to be pronounced grazers because of their dental structure with flat chewing surfaces on the molar-like teeth. This is also supported by the high (hypsodont) tooth crowns and the wide mouth with numerous shapes. The ungulates are mostly used as analogous examples, in which shapes with high tooth crowns and broad-lipped mouths are usually grass-eating, such as various cattle, horses or the white rhinoceros. In contrast, those with low tooth crowns and narrow snouts such as the duiker or the black rhinoceros feed largely selective from various leaves and other soft vegetable foods. In contrast to other large mylodontid sloths such as Glossotherium, Paramylodon or Lestodon, the mouth of Mylodon is relatively narrow. A special feature is the closed nasal arch, which is heavily roughened in its front area and thus offers muscle attachment points for a mobile upper lip. Something similar can be said about individual depressions in the vicinity of the infraorbital foramen, which also functioned as starting points for individual muscle strands in the nose and lip area. Maybe Mylodon was more well-adapted to a mixed-vegetation diet, which was picked up with the help of a movable upper lip. The loss of the front teeth in the upper row of teeth also leads to the assumption that, comparable to cattle, there was a horn-like structure on the middle jawbone that could be used to pluck the food.[34][14]
The entire anterior cranial structure of Mylodon is relatively solid, combined with a partially ossified nasal septum, it can be assumed that relatively high chewing forces acted when the food was chopped up. In contrast to the sometimes huge representatives of the Megatheriidae, the joint between the lower jaw and the skull of the Mylodonts was relatively low, roughly at the chewing level of the teeth. The resulting decreasing lever arm of the masseter muscle experiences through the structure of the zygomatic arch, mainly of the descending process, a certain compensation, so that there should have been only minor differences to the Megatheria with regard to the biting force. The extended mandibular joint allows a wide freedom of movement when chewing. Against this, however, is the zygomatic arch, which is not closed and therefore could only withstand the opposing forces of the masseter and musculus pterygoideus to a limited extent. It can therefore be assumed that forwards and backwards directed chewing movements dominated in Mylodon.[34][14] The flat tooth crowns lead to a comparatively small size of the total available chewing surface. In Mylodon, this amounts to a good 1320 mm² corresponding to other mylodonts of the same size. The Indian rhinoceros, which is comparable in terms of its dimensions, has, on the other hand, double to four times the value with 2660 to 5190 mm². The situation is similar with the hippopotamus , the total surface area of which is between 3290 and 5410 mm². The small total occlusal surface of the teeth in Mylodon probably resulted in a rather low processing capacity for the food in the mouth. This can result in either a high rate of fermentation in the gastrointestinal tract and/or a very slow metabolism concluded. The latter is the case with today's sloths. This is due to the long passage time of the food of up to a week through the large, multi-chambered stomach. It can be assumed that this also applies to the extinct sloths. Possibly this made the stomach of the mylodonts a functional equivalent to the complex stomach of the ruminants, whereby a long passage time of the food enabled efficient digestion, in which even more difficult to access nutrients could be provided, for example from foods with a greater fiber content. Such a digestive system could reduce the amount of processed food in the mouth and thus ultimately also the small total sales area with Mylodonhave balanced.[35][36]

Direct analysis of the food resources used is possible, among many other things, due to the numerous dung residues in the form of coprolites. These are available not only from the Cueva del Milodón in the Chilean part of Patagonia, but also from other caves. The coprolites in Mylodon have a diameter of up to 18 cm.[37] Investigations of the plant residues showed 80 to 95% sweet grasses and 5 to 20% sour grasses. Herbaceous plants, on the other hand, could only be detected in traces. Accordingly, Mylodon led, at least in southwestern Patagonia, a diet consisting almost exclusively of grasses. The food is reflected in the paleohabitat, as pollen analyzes show that the landscape at that time was tundra-like in character and was therefore almost free of trees with only a few low bushes. Occasional evidence of false beeches is interpreted as pollen carried by the wind. [38][39][40]
Locomotion
In general, large mylodonts are ground-dwelling animals. The lower section of the hind leg, which is very short compared to the upper, is also found in Mylodon, whose tibia is 27 cm in length and only half as long as the thigh bone, 59 cm in length. In comparison, the Megatheriidae possess significantly longer lower leg portions, about the almost equally-proportioned Pyramiodontherium possessing to a 47 cm long shin to a 49 cm long femur. Possibly these differences in the hind leg structure result in much more agile locomotion in the Megatheria in relation to the Mylodonts. [41] Similar to other large ground sloths, the hand of Mylodon made contact with the ground with the outer side edge and thus sat up rotated. This is indicated by the long metacarpal bones of the external digits and the decreasing number of phalanges on them. The special hand position protected the long claws of the inner digits, which did not penetrate the ground while walking. A functionally similar but fundamentally different hand position can be found in the ankle duct of the distantly related present-day great anteater. The elbow joint was pointed slightly outwards when standing on four feet and the arms were thus angled slightly inwards, which is evident from the position of the olecranon yields. The hands came to rest slightly within the width of the elbow. Such an orientation of the arms can effectively support the large mass of Mylodon. As a result, the hands would also be in a line with the feet, which is also conveyed, among other things, by footprints from Paramylodon. The laterally limited articular surface of the femoral head severely restricted the mobility of the hiindlimbs. The same applies to the forearm, the straight spoke with the laterally elongated head of which did not allow any major rotational movements. These features can be interpreted as adaptations to a purely terrestrial lifestyle. Finally, the muscle attachment points on the first cervical vertebra referenced, which are more developed than for example with Paramylodon. Correspondingly, the occipital joint surfaces are also somewhat further apart. Both can be interpreted as meaning that the more massive skull of Mylodon, caused by the lengthening of the snout region, required greater muscle support.[17][18]
For some of the mylodonts of South America, such as Glossotherium, a partially burrowing way of life is being reconstructed, which results from the construction of the foreleg, among other things. An indicator for this is the upper articular process (olecranon) of the ulna. The longer the olecranon, the higher the leverage of the forearm, since more attachment surface is available for the forearm muscles. In Glossotherium, the olecranon takes up up to 35% of the total length of the ulna. The resulting ability to dig would be comparable to that of the Tolypeutes armadillos, which seldom build their own burrows, but are able to do so.[42] The previous analyses for Mylodon resulted in a much shorter olecranon, which accounts for only about 22% of the total length of the ulna. However, the fact that proportional estimates for Mylodon refer to a not fully grown specimen is problematic.[43] Other clues can be derived from the construction of the hand. In Mylodon, for example, the metacarpal bones of the second and third ray are very delicate, in contrast to Glossotherium. A weakly pronounced central ray does not seem to support a digging activity, as this is usually most strongly developed in underground mammals. However, the distal articular facet of the third metacarpal bone is flat, which means that the middle finger is generally stiff and stable. The same articulation surface on the second metacarpal is significantly more rounded and thus supports greater mobility of the finger when gripping. This obviously resulted in functional differences between the individual rays of the hand. The rare signs of wear and tear on the last phalanx, which are isolated from the Cueva del Milodón several times, can serve as an additional indicator of digging activities.[18][43]
Predation and Parasites
Especially in southern and southwestern Patagonia, numerous bone changes in finds of Mylodon can be proven to be caused by predatory animals. This includes, above all, the remains from the Cueva del Milodón in southwestern Chile. Some caves in their immediate vicinity, such as Cueva Lago Sofía 4 and Cueva Chica, are interpreted as clumps of predators. [44][45] The same applies to the Cueva del Puma or the Cueva Fell in the Pali-Aike area of southern Chile. Some of the caves mainly contain smaller skeletal elements such as hand and foot bones or bone plates, which indicate that only part of the carcass was carried into the shelter. Whether this is the result of direct foraging or scavenging cannot be determined in many cases. Other caves, in turn, contained a larger proportion of young Mylodon animals.[46][47] The largest predators occurring at that time are the puma and the jaguar, as well as the sabre-toothed cat Smilodon populator and the extinct bear Arctotherium. The latter two could have reconstructed body weights of over 400 kg, with prey sizes between 1 and 2 t being assumed for the saber-toothed cat, which makes Smilodon a likely predator of Mylodon.[48][46][47][49]
In various coprolites produced by Mylodon, eggs of nematodes are preserved. The eggs are ovaloid in shape with lengths of almost 50 µm in length and 29 µm in thickness.[50] In addition, individual beetles could be detected.[37]
References
- Fiedal, Stuart (2009). "Sudden Deaths: The Chronology of Terminal Pleistocene Megafaunal Extinction" (PDF). In Haynes, Gary (ed.). American Megafaunal Extinctions at the End of the Pleistocene. Springer. pp. 21–37. doi:10.1007/978-1-4020-8793-6_2. ISBN 978-1-4020-8792-9.
- R. Owen (1840). Zoology of the Voyage of the Beagle. Part 1, Fossil Mammalia. Pp. 63-73.
- "Patagonia; Hesketh-Prichard's Stirring Tale of Exploration in the Far South". The New York Times. 20 December 1902. Retrieved 2008-11-22.
- Mylodon at Fossilworks.org
- C. Michael Hogan (2008) Cueva del Milodon, Megalithic Portal
- Bell, C. M. (2002). "Did elephants hang from trees? - the giant sloths of South America". Geology Today. 18 (2): 63–66 (see p. 66). doi:10.1046/j.1365-2451.2002.00334.x.
- Roosevelt, T.R. (1915-01-04). "Letter from Theodore Roosevelt to George Herbert Sherwood". theodorerooseveltcenter.org. Dickinson State University. Retrieved 2019-10-12.
- "Roosevelt Collections". amnh.org/exhibitions. AMNH. Retrieved 2019-10-12.
- Warren, D. (2016-05-28). "The ground sloth". Essays in Idleness. Retrieved 2019-10-12.
- Per Christiansen and Richard A. Fariña; 2003 "Mass estimation of two fossil ground sloths (Mammalia, Xenarthra, Mylodontidae)." Senckenbergiana Biologica. 83 (1), 95-101
- Analia M. Forasiepi, Agustin Martinelli and Jorge Blanco; 2007 "Fossil Bestiary: Mammals of Pleistocene Argentina." Buenos Aires. 1–181 (pp. 60–61)
- Diego Brandoni, Brenda S. Ferrero and Ernesto Brunetto. Mylodon darwini Owen (Xenarthra, Mylodontinae) from the Late Pleistocene of Mesopotamia, Argentina, with Remarks on Individual Variability, Paleobiology, Paleobiogeography, and Paleoenvironment. Journal of Vertebrate Paleontology 30(5):1547-1558. 2010 doi:10.1080/02724634.2010.501449 September 2010.
- Luciano Brambilla and Damián A. Ibarra: The occipital region of late Pleistocene Mylodontidae of Argentina. Boletín del Instituto de Fisiografía y Geología 88, 2018, pp. 1–9
- M. Susana Bargo and Sergio F. Vizcaíno: Paleobiology of Pleistocene ground sloths (Xenarthra, Tardigrada): biomechanics, morphogeometry and ecomorphology applied to the masticatory apparatus. Ameghiniana 45 (1), 2008, pp. 175-196
- McAfee, R.K. (2007). Reassessing the Taxonomy and Affinities of the Myodontinae sloths, Glossotherium and Paramylodon (Mammalia: Xenarthra: Tardigrada). Ph.D. Dissertation, Northern Illinois University.
- M. Susana Bargo, Gerardo De Iuliis and Sergio F. Vizcaíno; 2006 "Hypsodonty in Pleistocene ground sloths." Acta Palaeontologica Polonica. 51 (1): 53-61
- Lucas Kraglievich: Contribución al conocimiento de Mylodon darwini Owen y especies afines. Revista del Museo de La Plata 34, 1934, pp. 255-292
- Robert K. McAfee: Description of New Postcranial Elements of Mylodon darwinii Owen 1839 (Mammalia: Pilosa: Mylodontinae), and Functional Morphology of the Forelimb. Ameghiniana 53 (4), 2016, pp. 418-443
- José A. Haro, Adan A. Tauber and Jerónimo M. Krapovickas: Thoracic member (pectoral girdle and forelimb) bones of Mylodon darwinii Owen (Xenarthra, Mylodontidae) from the Late Pleistocene of Central Argentina and their phylogenetic implications. Paläontologische Zeitschrift 91, 2017, pp. 439–457, doi: 10.1007 / s12542-017-0350-z
- Francesco P. Moreno and Arthur Smith Woodward: On a Portion of Mammalian Skin, named Neomylodon listai, from a Cave near Consuelo Cove, Last Hope Inlet, Patagonia. Proceedings of the Zoological Society 1899, pp. 144–156
- Otto Nordenskjöld (with the participation of other authors): Scientific results of the Swedish expedition to the Magellan lands 1895-1897, under the direction of Dr. Otto Nordenskjöld. Volume II: Zoology. Stockholm, 1899, pp. 1–170 (especially pp. 149–170)
- Einar Lönnberg: On a remarkable piece of skin from Cueva Eberhardt, Last Hope Inlet, Patagonia. Proceedings of the Zoological Society 1900, pp. 379-383
- Arthur Smith Woodward: On some remains of Grypotherium (Neomylodon) listai and associated mammals from a cavern near Consuelo Cove, Patagonia. Proceedings of the Zoological Society 1900, pp. 64–79
- RG Ridewood: On the Structure of the Hairs of Mylodon Listai and other South American Edentata. Quaternary Review of Microscopic Science 44, 1901, pp. 393-411
- Hermann Burmeister: skin armor at Mylodon. Archives for anatomy, physiology and scientific medicine 1865, pp. 334–336
- Hermann Burmeister: Fauna Argentina. Primera party. Mamiferos fósiles. Lista de los mamiferos fósiles del terreno diluviano. Anales del Museo Público de Buenos Aires 1, 1867, pp. 87–300 (p. 173)
- Robert V. Hill: Comparative Anatomy and Histology of Xenarthran Osteoderms. Journal of Morphology 267, 2005, pp. 1441-1460
- Patricio López-Mendoza and Francisco Mena-Larraín: Extinct ground sloth dermal bones and their role in the taphonomic research of caves: the case of Baño Nuevo-1 (Andean Central Patagonia, Chile). Revista Mexicana de Ciencias Geológicas 28, 2011, pp. 519-532
- Wilhelm Branco: The application of X-rays in paleontology. Treatises of the Royal Prussian Academy of Sciences Berlin 1906, pp. 1–55
- H. Gregory McDonald: An Overview of the Presence of Osteoderms in Sloths: Implications for Osteoderms as a Plesiomorphic Character of the Xenarthra. Journal of Mammalogy 25, 2018, pp. 485-493, doi: 10.1007 / s10914-017-9415-8
- Alberto Boscaini, François Pujos and Timothy J. Gaudin: A reappraisal of the phylogeny of Mylodontidae (Mammalia, Xenarthra) and the divergence of mylodontine and lestodontine sloths. Zoologica Scripta 48 (6), 2019, pp. 691-710, doi: 10.1111 / zsc.12376
- Presslee, S.; Slater, G. J.; Pujos, F.; Forasiepi, A. M.; Fischer, R.; Molloy, K.; Mackie, M.; Olsen, J. V.; Kramarz, A.; Taglioretti, M.; Scaglia, F.; Lezcano, M.; Lanata, J. L.; Southon, J.; Feranec, R.; Bloch, J.; Hajduk, A.; Martin, F. M.; Gismondi, R. S.; Reguero, M.; de Muizon, C.; Greenwood, A.; Chait, B. T.; Penkman, K.; Collins, M.; MacPhee, R.D.E. (2019). "Palaeoproteomics resolves sloth relationships". Nature Ecology & Evolution. 3 (7): 1121–1130. doi:10.1038/s41559-019-0909-z. PMID 31171860.
- Delsuc, F.; Kuch, M.; Gibb, G. C.; Karpinski, E.; Hackenberger, D.; Szpak, P.; Martínez, J. G.; Mead, J. I.; McDonald, H. G.; MacPhee, R.D.E.; Billet, G.; Hautier, L.; Poinar, H. N. (2019). "Ancient Mitogenomes Reveal the Evolutionary History and Biogeography of Sloths". Current Biology. 29 (12): 2031–2042.e6. doi:10.1016/j.cub.2019.05.043. PMID 31178321.
- M. Susana Bargo, Néstor Toledo and Sergio F. Vizcaíno: Muzzle of South American Pleistocene Ground Sloths (Xenarthra, Tardigrada). Journal of Morphology 267, 2006, pp. 248-263
- Sergio F. Vizcaíno, M. Susana Bargo and Guillermo H. Cassini: Dental occlusal surface area in relation to body mass, food habits and other biological features in fossil xenarthrans. Ameghiniana 43 (1), 2006, pp. 11-26
- Sergio F. Vizcaíno: The teeth of the “toothless”: novelties and key innovations in the evolution of xenarthrans (Mammalia, Xenarthra). Paleobiology 35 (3), 2009, pp. 343-366
- Luis Alberto Borrero and Fabiana María Martin: Taphonomic observations on ground sloth bone and dung from Cueva del Milodón, Ultima Esperanza, Chile: 100 years of research history. Quaternary International 278, 2012, pp. 3-11
- Vera Markgraf: Late Pleistocene Faunal Extinctions in Southern Patagonia. Science 228, 1985, pp. 1110-409
- Calvin J. Heusser, Luis A. Borrero and José A. Lanata: Late Glacial vegetation at Cueva del Mylodon. Anales del Instituto de la Patagonia (Ciencias Naturales series) 21, 1992, pp. 97-102
- Rodrigo Villa-Martínez and Patricio I. Moreno: Pollen evidence for variations in the southern margin of the westerly winds in SW Patagonia over the last 12,600 years. Quaternary Research 68, 2007, pp. 400-409
- Gerardo De Iuliis, Guillermo H. Ré and Sergio F. Vizcaíno: The Toro Negro megatheriine (Mammalia, Xenarthra): A new species of Pyramiodontherium and a review of Plesiomegatherium. Journal of Vertebrate Paleontology 24 (1), 2004, pp. 214-227
- M. Susana Bargo, Sergio F. Vizcaíno, Fernando M. Archuby and R. Ernesto Blanco: Limb bone proportions, strength and digging in some Lujanian (Late Pleistocene-Early Holocene) mylodontid ground sloths (Mammalia, Xenarthra). Journal of Vertebrate Paleontology 20 (3), 2000, pp. 601-610
- José A. Haro, Adan A. Tauber and Jerónimo M. Krapovickas: The Manus of Mylodon darwinii Owen (Tardigrada, Mylodontidae) and Ist Phylogenetic Implications. Journal of Vertebrate Paleontology 36 (5), 2016, p. E1188824, doi: 10.1080 / 02724634.2016.1188824
- Luis A. Borrero, Fabiana M. Martin and Alfredo Prieto: La Cueva Lago Sofía 4, Ultima Esperanza, Chile: U madriguera felina del Pleistoceno tardío. Anales del Instituto de la Patagonia 25, 1997, pp. 103-122
- Fabiana Martin, Manuel San Román, Flavia Morello, Dominique Todisco, Francisco J. Prevosti and Luis A. Borrero: Land of the ground sloths: Recent research at Cueva Chica, Ultima Esperanza, Chile. Quaternary International 305 (2013) 56-66
- Fabiana M. Martin: Bone crunching felids at the end of the Pleistocene in Fuego-Patagonia, Chile. Journal of Taphonomy 6 (3/4), 2008, pp. 337-372
- Luis Alberto Borrero: The Elusive Evidence: The Archeological Record of the South American Extinct Megafauna. In: G. Haynes (Ed.): American Megafaunal Extinctions at the End of the Pleistocene. Springer Science + Business Media BV, 2009, pp. 145-168
- Aldo Manzuetti, Daniel Perea, Washington Jones, Martín Ubilla and Andrés Rinderknecht: An extremely large saber-tooth cat skull from Uruguay (late Pleistocene – early Holocene, Dolores Formation): body size and paleobiological implications. Alcheringa: An Australasian Journal of Palaeontology, 2020, doi: 10.1080
- Francisco J. Prevosti and Fabiana M. Martin, Paleoecology of the mammalian predator guild of Southern Patagonia during the latest Pleistocene: Ecomorphology, stable isotopes, and taphonomy. Quaternary International 305, 2013, pp. 74-84
- Raúl A. Ringuelet: Restos de probables huevos de nematodes en el estiercol del edentado extinguido Mylodon listai (Ameghino). Ameghiniana 1 (1/2); 1957, pp. 15-16