Ocean chemistry
Ocean chemistry, also known as marine chemistry, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.
| Component | Concentration (mol/kg) |
|---|---|
| H 2O | 53.6 |
| Cl− | 0.546 |
| Na+ | 0.469 |
| Mg2+ | 0.0528 |
| SO2− 4 | 0.0282 |
| Ca2+ | 0.0103 |
| K+ | 0.0102 |
| CT | 0.00206 |
| Br− | 0.000844 |
| BT (total boron) | 0.000416 |
| Sr2+ | 0.000091 |
| F− | 0.000068 |
The impact of human activity on the chemistry of the earth's oceans has increased over time, with pollution from industry and various land-use practices significantly affecting the oceans. Moreover, increasing levels of carbon dioxide in the earth's atmosphere have led to ocean acidification, which has negative effects on marine ecosystems. The international community has agreed that restoring the chemistry of the oceans is a priority, and efforts toward this goal are tracked as part of Sustainable Development Goal 14.
Marine chemistry on earth
Organic compounds in the oceans
Colored dissolved organic matter (CDOM) is estimated to range 20-70% of carbon content of the oceans, being higher near river outlets and lower in the open ocean.[2]
Marine life is largely similar in biochemistry to terrestrial organisms, except that they inhabit a saline environment. One consequence of their adaptation is that marine organisms are the most prolific source of halogenated organic compounds.[3]
Chemical ecology of extremophiles
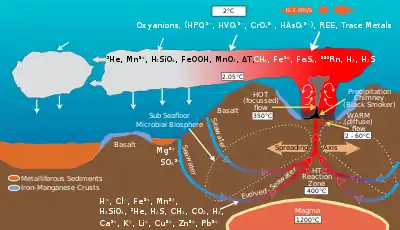
The ocean provides special marine environments inhabited by extremophiles that thrive under unusual conditions of temperature, pressure, and darkness. Such environments include hydrothermal vents and black smokers and cold seeps on the ocean floor, with entire ecosystems of organisms that have a symbiotic relationship with compounds that provided energy through a process called chemosynthesis.
Plate tectonics
Seafloor spreading on mid-ocean ridges is a global scale ion-exchange system.[4] Hydrothermal vents at spreading centers introduce various amounts of iron, sulfur, manganese, silicon and other elements into the ocean, some of which are recycled into the ocean crust. Helium-3, an isotope that accompanies volcanism from the mantle, is emitted by hydrothermal vents and can be detected in plumes within the ocean.[5]
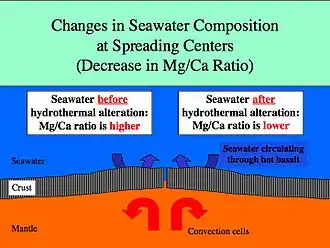
Spreading rates on mid-ocean ridges vary between 10 and 200 mm/yr. Rapid spreading rates cause increased basalt reactions with seawater. The magnesium/calcium ratio will be lower because more magnesium ions are being removed from seawater and consumed by the rock, and more calcium ions are being removed from the rock and released to seawater. Hydrothermal activity at ridge crest is efficient in removing magnesium.[6] A lower Mg/Ca ratio favors the precipitation of low-Mg calcite polymorphs of calcium carbonate (calcite seas).[4]
Slow spreading at mid-ocean ridges has the opposite effect and will result in a higher Mg/Ca ratio favoring the precipitation of aragonite and high-Mg calcite polymorphs of calcium carbonate (aragonite seas).[4]
Experiments show that most modern high-Mg calcite organisms would have been low-Mg calcite in past calcite seas,[7] meaning that the Mg/Ca ratio in an organism's skeleton varies with the Mg/Ca ratio of the seawater in which it was grown.
The mineralogy of reef-building and sediment-producing organisms is thus regulated by chemical reactions occurring along the mid-ocean ridge, the rate of which is controlled by the rate of sea-floor spreading.[6][7]
Human impacts

Marine pollution occurs when harmful effects result from the entry into the ocean of chemicals, particles, industrial, agricultural and residential waste, noise, or the spread of invasive organisms. Eighty percent of marine pollution comes from land. Air pollution is also a contributing factor by carrying off iron, carbonic acid, nitrogen, silicon, sulfur, pesticides or dust particles into the ocean.[8] Land and air pollution have proven to be harmful to marine life and its habitats.[9]
The pollution often comes from nonpoint sources such as agricultural runoff, wind-blown debris, and dust. Pollution in large bodies of water can be aggravated by physical phenomena like the biological effects of Langmuir circulation. Nutrient pollution, a form of water pollution, refers to contamination by excessive inputs of nutrients. It is a primary cause of eutrophication of surface waters, in which excess nutrients, usually nitrates or phosphates, stimulate algae growth. Many potentially toxic chemicals adhere to tiny particles which are then taken up by plankton and benthic animals, most of which are either deposit feeders or filter feeders. In this way, the toxins are concentrated upward within ocean food chains. Many particles combine chemically in a manner highly depletive of oxygen, causing estuaries to become anoxic.
When pesticides are incorporated into the marine ecosystem, they quickly become absorbed into marine food webs. Once in the food webs, these pesticides can cause mutations, as well as diseases, which can be harmful to humans as well as the entire food web. Toxic metals can also be introduced into marine food webs. These can cause a change to tissue matter, biochemistry, behaviour, reproduction, and suppress growth in marine life. Also, many animal feeds have a high fish meal or fish hydrolysate content. In this way, marine toxins can be transferred to land animals, and appear later in meat and dairy products.
In order to protect the ocean from marine pollution, policies have been developed internationally. The international community has agreed that reducing pollution in the oceans is a priority, which is tracked as part of Sustainable Development Goal 14 which actively seeks to undo these human impacts on the oceans. There are different ways for the ocean to get polluted, therefore there have been multiple laws, policies, and treaties put into place throughout history.Climate change
Increased carbon dioxide levels, resulting from anthropogenic factors or otherwise, have the potential to impact ocean chemistry. Global warming and changes in salinity have significant implications for ecology of marine environments.[10] One proposal suggests dumping massive amounts of lime, a base, to reverse the acidification and "increase the sea's ability to absorb carbon dioxide from the atmosphere".[11][12][13]
Ocean acidification
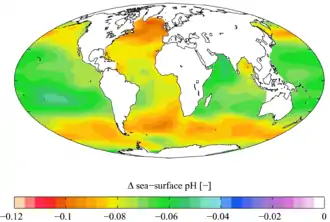
2 between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas

2 on transect line 5 off Pt. St. George, California. The potential density surfaces are superimposed on the temperature section. The 26.2 potential density surface delineates the location of the first instance in which the undersaturated water is upwelled from depths of 150 to 200 m onto the shelf and outcropping at the surface near the coast. The red dots represent sample locations.[14]
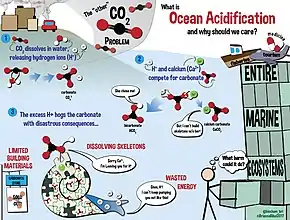
Ocean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO
2) from the atmosphere.[15] The main cause of ocean acidification is the burning of fossil fuels. Seawater is slightly basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7).[16] The issue of ocean acidification is the decreased production of the shells of shellfish and other aquatic life with calcium carbonate shells. The calcium carbonate shells can not reproduce under high saturated acidotic waters. An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes.[17][18] Some of it reacts with the water to form carbonic acid. Some of the resulting carbonic acid molecules dissociate into a bicarbonate ion and a hydrogen ion, thus increasing ocean acidity (H+ ion concentration). Between 1751 and 1996, surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14,[19] representing an increase of almost 30% in H+ ion concentration in the world's oceans.[20][21] Earth System Models project that, by around 2008, ocean acidity exceeded historical analogues[22] and, in combination with other ocean biogeochemical changes, could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean beginning as early as 2100.[23]
Increasing acidity is thought to have a range of potentially harmful consequences for marine organisms such as depressing metabolic rates and immune responses in some organisms and causing coral bleaching.[24] By increasing the presence of free hydrogen ions, the additional carbonic acid that forms in the oceans ultimately results in the conversion of carbonate ions into bicarbonate ions. Ocean alkalinity (roughly equal to [HCO3−] + 2[CO32−]) is not changed by the process, or may increase over long time periods due to carbonate dissolution.[25] This net decrease in the amount of carbonate ions available may make it more difficult for marine calcifying organisms, such as coral and some plankton, to form biogenic calcium carbonate, and such structures become vulnerable to dissolution.[26] Ongoing acidification of the oceans may threaten future food chains linked with the oceans.[27][28] As members of the InterAcademy Panel, 105 science academies have issued a statement on ocean acidification recommending that by 2050, global CO
2 emissions be reduced by at least 50% compared to the 1990 level.[29] To ensure that ocean acidification is minimized, the United Nation's Sustainable Development Goal 14 ("Life below Water") aims to ensure that oceans are conserved and sustainably used.[30]
Latest research challenges the potential negative impact of end-of-century ocean acidification level on the coral fish behavior and suggests that the effect could be negligible.[31] Controversially, laboratory experiments in the controlled environment showed CO
2 induced growth of the phytoplankton species.[32] Field study of the coral reef in Queensland and Western Australia from 2007 to 2012 argues that corals are more resistant to the environmental pH changes than previously thought, due to internal homeostasis regulation; this makes thermal change, rather than acidification, the main factor for coral reef vulnerability due to global warming.[33]
While ongoing ocean acidification is at least partially anthropogenic in origin, it has occurred previously in Earth's history,[34] and the resulting ecological collapse in the oceans had long-lasting effects for global carbon cycling and climate.[35][36] The most notable example is the Paleocene-Eocene Thermal Maximum (PETM),[37] which occurred approximately 56 million years ago when massive amounts of carbon entered the ocean and atmosphere, and led to the dissolution of carbonate sediments in all ocean basins.
Ocean acidification has been compared to anthropogenic climate change and called the "evil twin of global warming"[38][39][40][41][42] and "the other CO2 problem".[39][41][43] Freshwater bodies also appear to be acidifying, although this is a more complex and less obvious phenomenon.[44][45]
Marine chemistry on other planets and their moons
A planetary scientist using data from the Cassini spacecraft has been researching the marine chemistry of Saturn's moon Enceladus using geochemical models to look at changes through time.[46] The presence of salts may indicate a liquid ocean within the moon, raising the possibility of the existence of life, "or at least for the chemical precursors for organic life".[46][47]
See also
References
- DOE (1994). "5" (PDF). In A.G. Dickson; C. Goyet (eds.). Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water. 2. ORNL/CDIAC-74.
- Coble, Paula G. (2007). "Marine Optical Biogeochemistry: The Chemistry of Ocean Color". Chemical Reviews. 107 (2): 402–418. doi:10.1021/cr050350+. PMID 17256912.
- Gribble, Gordon W. (2004). "Natural Organohalogens: A New Frontier for Medicinal Agents?". Journal of Chemical Education. 81 (10): 1441. Bibcode:2004JChEd..81.1441G. doi:10.1021/ed081p1441.
- Stanley, S.M.; Hardie, L.A. (1999). "Hypercalcification: paleontology links plate tectonics and geochemistry to sedimentology". GSA Today. 9 (2): 1–7.
- Lupton, John (1998-07-15). "Hydrothermal helium plumes in the Pacific Ocean". Journal of Geophysical Research: Oceans. 103 (C8): 15853–15868. Bibcode:1998JGR...10315853L. doi:10.1029/98jc00146. ISSN 0148-0227.
- Coggon, R. M.; Teagle, D. A. H.; Smith-Duque, C. E.; Alt, J. C.; Cooper, M. J. (2010-02-26). "Reconstructing Past Seawater Mg/Ca and Sr/Ca from Mid-Ocean Ridge Flank Calcium Carbonate Veins". Science. 327 (5969): 1114–1117. Bibcode:2010Sci...327.1114C. doi:10.1126/science.1182252. ISSN 0036-8075. PMID 20133522. S2CID 22739139.
- Ries, Justin B. (2004). "Effect of ambient Mg/Ca ratio on Mg fractionation in calcareous marine invertebrates: A record of the oceanic Mg/Ca ratio over the Phanerozoic". Geology. 32 (11): 981. Bibcode:2004Geo....32..981R. doi:10.1130/G20851.1. ISSN 0091-7613.
- Duce, Robert, Galloway, J. and Liss, P. (2009). "The Impacts of Atmospheric Deposition to the Ocean on Marine Ecosystems and Climate WMO Bulletin Vol 58 (1)". Retrieved September 22, 2020.
- "What is the biggest source of pollution in the ocean?". National Ocean Service.
- Millero, Frank J. (2007). "The Marine Inorganic Carbon Cycle". Chemical Reviews. 107 (2): 308–341. doi:10.1021/cr0503557. PMID 17300138.
- Clark, Duncan (2009-07-12). "Cquestrate: adding lime to the oceans". The Guardian. ISSN 0261-3077. Retrieved 2019-07-16.
- Katz, Ian (2009-07-12). "Twenty ideas that could save the world". The Guardian. ISSN 0261-3077. Retrieved 2019-07-16.
- http://www.infrastructurist.com/2009/07/14/from-the-uk-20-bold-schemes-that-could-save-us-from-global-warming/ Archived 2009-07-18 at the Wayback Machine July 14, 2009 Infrastructurist
- Feely, R. A.; Sabine, C. L.; Hernandez-Ayon, J. M.; Ianson, D.; Hales, B. (June 2008). "Evidence for upwelling of corrosive "acidified" water onto the continental shelf". Science. 320 (5882): 1490–2. Bibcode:2008Sci...320.1490F. CiteSeerX 10.1.1.328.3181. doi:10.1126/science.1155676. PMID 18497259. S2CID 35487689. Retrieved 2014-01-25 – via Pacific Marine Environmental Laboratory (PMEL).
- Caldeira, K.; Wickett, M. E. (2003). "Anthropogenic carbon and ocean pH". Nature. 425 (6956): 365. Bibcode:2001AGUFMOS11C0385C. doi:10.1038/425365a. PMID 14508477. S2CID 4417880.
- The ocean would not become acidic even if it were to absorb the CO2 produced from the combustion of all fossil fuel resources.
- Millero, Frank J. (1995). "Thermodynamics of the carbon dioxide system in the oceans". Geochimica et Cosmochimica Acta. 59 (4): 661–677. Bibcode:1995GeCoA..59..661M. doi:10.1016/0016-7037(94)00354-O.
- Feely, R. A.; Sabine, C. L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V. J.; Millero, F. J. (July 2004). "Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans". Science. 305 (5682): 362–366. Bibcode:2004Sci...305..362F. doi:10.1126/science.1097329. PMID 15256664. S2CID 31054160. Retrieved 2014-01-25 – via Pacific Marine Environmental Laboratory (PMEL).
- Jacobson, M. Z. (2005). "Studying ocean acidification with conservative, stable numerical schemes for nonequilibrium air-ocean exchange and ocean equilibrium chemistry". Journal of Geophysical Research: Atmospheres. 110: D07302. Bibcode:2005JGRD..11007302J. doi:10.1029/2004JD005220.
- Hall-Spencer, J. M.; Rodolfo-Metalpa, R.; Martin, S.; et al. (July 2008). "Volcanic carbon dioxide vents show ecosystem effects of ocean acidification". Nature. 454 (7200): 96–9. Bibcode:2008Natur.454...96H. doi:10.1038/nature07051. hdl:10026.1/1345. PMID 18536730. S2CID 9375062.
- "Report of the Ocean Acidification and Oxygen Working Group, International Council for Science's Scientific Committee on Ocean Research (SCOR) Biological Observatories Workshop" (PDF).
- Mora, C (2013). "The projected timing of climate departure from recent variability". Nature. 502 (7470): 183–187. Bibcode:2013Natur.502..183M. doi:10.1038/nature12540. PMID 24108050. S2CID 4471413.
Global mean ocean pH moved outside its historical variability by 2008 (±3 years s.d.), regardless of the emissions scenario analysed
- Mora, C.; et al. (2013). "Biotic and Human Vulnerability to Projected Changes in Ocean Biogeochemistry over the 21st Century". PLOS Biology. 11 (10): e1001682. doi:10.1371/journal.pbio.1001682. PMC 3797030. PMID 24143135.
- Anthony, KRN; et al. (2008). "Ocean acidification causes bleaching and productivity loss in coral reef builders". Proceedings of the National Academy of Sciences. 105 (45): 17442–17446. Bibcode:2008PNAS..10517442A. doi:10.1073/pnas.0804478105. PMC 2580748. PMID 18988740.
- Kump, L.R.; Bralower, T.J.; Ridgwell, A. (2009). "Ocean acidification in deep time". Oceanography. 22: 94–107. doi:10.5670/oceanog.2009.10. Retrieved 16 May 2016.
- Orr, James C.; et al. (2005). "Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms" (PDF). Nature. 437 (7059): 681–686. Bibcode:2005Natur.437..681O. doi:10.1038/nature04095. PMID 16193043. S2CID 4306199. Archived from the original (PDF) on 25 June 2008.
- Cornelia Dean (30 January 2009). "Rising Acidity Is Threatening Food Web of Oceans, Science Panel Says". New York Times.
- Robert E. Service (13 July 2012). "Rising Acidity Brings and Ocean Of Trouble". Science. 337 (6091): 146–148. Bibcode:2012Sci...337..146S. doi:10.1126/science.337.6091.146. PMID 22798578.
- IAP (June 2009). "Interacademy Panel (IAP) Member Academies Statement on Ocean Acidification"., Secretariat: TWAS (the Academy of Sciences for the Developing World), Trieste, Italy.
- "Goal 14 targets". UNDP. Retrieved 2020-09-24.
- Clark, Timothy D.; Raby, Graham D.; Roche, Dominique G.; Binning, Sandra A.; Speers-Roesch, Ben; Jutfelt, Fredrik; Sundin, Josefin (January 2020). "Ocean acidification does not impair the behaviour of coral reef fishes". Nature. 577 (7790): 370–375. Bibcode:2020Natur.577..370C. doi:10.1038/s41586-019-1903-y. ISSN 1476-4687. PMID 31915382. S2CID 210118722.
- Pardew, Jacob; Blanco Pimentel, Macarena; Low-Decarie, Etienne (April 2018). "Predictable ecological response to rising CO 2 of a community of marine phytoplankton". Ecology and Evolution. 8 (8): 4292–4302. doi:10.1002/ece3.3971. PMC 5916311. PMID 29721298.
- McCulloch, Malcolm T.; D’Olivo, Juan Pablo; Falter, James; Holcomb, Michael; Trotter, Julie A. (2017-05-30). "Coral calcification in a changing World and the interactive dynamics of pH and DIC upregulation". Nature Communications. 8 (1): 15686. Bibcode:2017NatCo...815686M. doi:10.1038/ncomms15686. ISSN 2041-1723. PMC 5499203. PMID 28555644.
- Zeebe, R.E. (2012). "History of Seawater Carbonate Chemistry, Atmospheric CO
2, and Ocean Acidification". Annual Review of Earth and Planetary Sciences. 40 (1): 141–165. Bibcode:2012AREPS..40..141Z. doi:10.1146/annurev-earth-042711-105521. S2CID 18682623. - Henehan, Michael J.; Ridgwell, Andy; Thomas, Ellen; Zhang, Shuang; Alegret, Laia; Schmidt, Daniela N.; Rae, James W. B.; Witts, James D.; Landman, Neil H.; Greene, Sarah E.; Huber, Brian T. (2019-10-17). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. ISSN 0027-8424. PMC 6842625. PMID 31636204.
- Carrington, Damian (2019-10-21). "Ocean acidification can cause mass extinctions, fossils reveal". The Guardian. ISSN 0261-3077. Retrieved 2019-10-22.
- Zachos, J.C.; Röhl, U.; Schellenberg, S.A.; Sluijs, A.; Hodell, D.A.; Kelly, D.C.; Thomas, E.; Nicolo, M.; Raffi, I.; Lourens, L. J.; McCarren, H.; Kroon, D. (2005). "Rapid acidification of the ocean during the Paleocene-Eocene thermal maximum". Science. 308 (5728): 1611–1615. Bibcode:2005Sci...308.1611Z. doi:10.1126/science.1109004. hdl:1874/385806. PMID 15947184. S2CID 26909706.
- "Ocean Acidification Is Climate Change's 'Equally Evil Twin,' NOAA Chief Says". Huffington Post. 9 July 2012. Archived from the original on 12 July 2012. Retrieved 2012-07-09.
- Nina Notman (29 July 2014). "The other carbon dioxide problem". Chemistry World.
- Alex Rogers (9 October 2013). "Global warming's evil twin: ocean acidification". The Conversation.
- Hennige, S.J. (2014). "Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to ocean acidification". Deep-Sea Research Part II. 99: 27–35. Bibcode:2014DSRII..99...27H. doi:10.1016/j.dsr2.2013.07.005.
- Pelejero, C. (2010). "Paleo-perspectives on ocean acidification". Trends in Ecology and Evolution. 25 (6): 332–344. doi:10.1016/j.tree.2010.02.002. PMID 20356649.
- Doney, S.C. (2009). "Ocean Acidification: The Other CO
2 Problem". Annual Review of Marine Science. 1: 169–192. Bibcode:2009ARMS....1..169D. doi:10.1146/annurev.marine.010908.163834. PMID 21141034. S2CID 402398. - Gies, E. (11 January 2018). "Like Oceans, Freshwater Is Also Acidifying". Scientific American. Retrieved 2018-01-13.
- Weiss, L. C.; Pötter, L.; Steiger, A.; Kruppert, S.; Frost, U.; Tollrian, R. (2018). "Rising pCO2 in Freshwater Ecosystems Has the Potential to Negatively Affect Predator-Induced Defenses in Daphnia". Current Biology. 28 (2): 327–332.e3. doi:10.1016/j.cub.2017.12.022. PMID 29337079.
- Pete Spotts Cassini spacecraft finds evidence for liquid water on Enceladus June 25, 2009 Christian Science Monitor
- Postberg, F.; Kempf, S.; Schmidt, J.; Brilliantov, N.; Beinsen, A.; Abel, B.; Buck, U.; Srama, R. (2009). "Sodium salts in E-ring ice grains from an ocean below the surface of Enceladus". Nature. 459 (7250): 1098–1101. Bibcode:2009Natur.459.1098P. doi:10.1038/nature08046. PMID 19553992. S2CID 205216877.