Pseudomyxoma peritonei
Pseudomyxoma peritonei (PMP) is a clinical condition caused by cancerous cells (mucinous adenocarcinoma) that produce abundant mucin or gelatinous ascites.[1] The tumors cause fibrosis of tissues and impede digestion or organ function, and if left untreated, the tumors and mucin they produce will fill the abdominal cavity. This will result in compression of organs and will destroy the function of the colon, small intestine, stomach, or other organs. Prognosis with treatment in many cases is optimistic,[2] but the disease is lethal if untreated, with death occurring via cachexia, bowel obstruction, or other types of complications.
| Pseudomyxoma peritonei | |
|---|---|
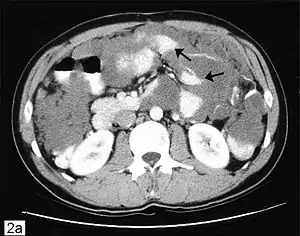 | |
| Computed tomographic scan of an abdomen showing pseudomyxoma peritonei with multiple peritoneal masses (arrow) with "scalloping effect" seen. | |
| Specialty | Oncology |
This disease is most commonly caused by an appendiceal primary cancer (cancer of the appendix); mucinous tumors of the ovary have also been implicated, although in most cases ovarian involvement is favored to be a metastasis from an appendiceal or other gastrointestinal source. Disease is typically classified as low- or high-grade (with signet ring cells). When disease presents with low-grade histologic features the cancer rarely spreads through the lymphatic system or through the bloodstream.
Signs and symptoms
Signs and symptoms of pseudomyxoma peritonei may include abdominal or pelvic pain and/or bloating, distension, digestive disorders, weight changes, increased girth, and infertility.
Cause
The primary tumor appears to arise from the MUC2 expressing goblet cells and most commonly from these cells in the appendix. The K-Ras and p53 genes may be involved in the oncogenesis. It may be diagnosed with a range of conditions. While the majority of these cases are associated with appendiceal carcinomas,[3] other conditions may also be found, including disseminated peritoneal adenomucinosis (DPAM), peritoneal carcinomas, several mucinous tumors (mucinous adenocarcinoma, mucinous cystadenoma, and mucinous cystadenocarcinoma), as well as other disease states.[4] Other primary sites that have been reported include colon, rectum, stomach, gallbladder, bile ducts, small intestine, urinary bladder, lung, breast, fallopian tubes, and the pancreas.
Diagnosis
This disease is often discovered during surgery for other conditions, e.g., hernia repair, following which an experienced pathologist can confirm the diagnosis. Advanced stages may present as tumors palpable on the abdomen or distention of the belly ("jelly belly" is sometimes used as a slang term for the condition). Due to the rarity of this disease, it is important to obtain an accurate diagnosis so that appropriate treatment may be obtained from a Gastro intestinal cancer surgeon. Diagnostic tests may include CT scans, examination of tissue samples obtained through laparoscopy, and the evaluation of tumor markers. In most cases a colonoscopy is unsuitable as a diagnostic tool because in most cases appendix cancer invades the abdominal cavity but not the colon (however, spread inside the colon is occasionally reported). PET scans may be used to evaluate high-grade mucinous adenocarcinoma, but this test is not reliable for detecting low-grade tumors because those do not take up the dye which shows up on scans. New MRI procedures are being developed for disease monitoring, but standard MRIs are not typically used as a diagnostic tool. Diagnosis is confirmed through pathology.
Classification
There is substantial debate regarding histopathologic classification of pseudomyxoma peritonei.[5] In 1995, Ronnett et al.[6] proposed separating pseudomyxoma peritonei cases into two diagnostic categories: adenoma (disseminated peritoneal adenomucinosis, DPAM) or carcinoma (peritoneal mucinous carcinomatosis, PMCA) with a third category reserved for cases with intermediate features. In this classification system, cases of DPAM were characterized by peritoneal lesions composed of abundant extracellular mucin containing scant simple to focally proliferative mucinous epithelium with little cytologic atypia or mitotic activity (in other words, most cells looked fairly normal and there was no evidence of mitosis which would indicate that cells were rapidly dividing), with or without an associated appendiceal mucinous adenoma. Cases of PMCA were characterized by peritoneal lesions composed of more abundant mucinous epithelium with the architectural and cytologic features of carcinoma (irregular cells, evidence that cells were rapidly dividing, and other criteria), with or without an associated primary mucinous adenocarcinoma. Bradley et al. (2007)[7] argued that continued use of non-malignant terms, i.e., adenoma, for those frequent cases with low-grade features (such as DPAM), is misleading because pseudomyxoma peritonei is a disease state that results from invasion of the abdominal cavity by cells with uncontrolled growth. Bradley states that an adenoma, by definition, is a tumor confined to the appendiceal mucosa with absolutely no evidence of invasion beyond the muscularis mucosae.
The term mucinous adenocarcinoma is used in different contexts depending on the reference material used by the pathologist for disease classification. For example, neoplasms characterized by high-grade features, invasive glands and or signet ring cells, are termed adenocarcinoma in pathology literature.[8] However, some pathologists (e.g., Odze and Goldblum, Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 2nd ed.) also use the term mucinous adenocarcinoma when referring to low-grade, well-differentiated tumors lacking high-grade features. Low-grade mucinous adenocarcinoma is used by the American Joint Committee on Cancer and World Health Organization and is nearly or completely synonymous with the DPAM designation.[9] For low-grade mucinous adenocarcinoma, disease may be designated as "benign" because tumors do not invade deeply into tissue and rarely metastasize to parenchyma of organs; this designation may be misleading and confusing to the layperson because pseudomyxoma peritonei is not a harmless condition, fatal if untreated. High-grade or poorly differentiated mucinous adenocarcinoma has a generally poorer prognosis, though surgical treatment with heated intra-peritoneal chemotherapy (HIPEC) is yielding promising outcomes (see surgical treatment).
Immunohistochemistry
Immunohistochemical features:
- Diffuse expression of SATB2, CK20, CDX2, and mCEA
- Sometimes patchy CK7; negative PAX8
- High-grade neoplasms may show loss of DPC4 (10%)[10]
Treatment
Treatment is variable, both due to its rarity and to its frequently slow-growing nature. Treatment ranges from watchful waiting to debulking and hyperthermic intraperitoneal chemotherapy (HIPEC, also called intraperitoneal hyperthermic chemotherapy, IPHC) with cytoreductive surgery.[11]
Surgical
The standard of care for mucinous adenocarcinoma with clinical condition PMP[12] involves cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC), performed by surgical oncologists who specialize in treating PMP. Some surgeons also apply early post-operative intraperitonial chemotherapy (EPIC), adjunct to surgical cytoreduction and HIPEC. In situations where surgery is not required immediately, patients can be monitored via CT scans, tumor marker laboratory tests, and physical symptoms, to determine when, and if, surgery is warranted. Although some surgical procedures may be rather extensive, patients can and do recover from surgery, and the majority of these patients can and do live productive lives.
In debulking, the surgeon attempts to remove as much tumor as possible. CRS or cytoreductive surgery involves surgical removal of the peritoneum and any adjacent organs which appear to have tumor seeding. Since the mucus tends to pool at the bottom of the abdominal cavity, it is common to remove the ovaries, fallopian tubes, uterus, and parts of the large intestine. Depending upon the spread of the tumor, other organs might be removed, including but not limited to the gallbladder, spleen, and portions of the small intestine and/or stomach. For organs that cannot be removed safely (like the liver), the surgeon strips off the tumor from the surface.[13]
Chemotherapy
Chemotherapy (typically utilising the chemotherapeutic agent Mitomycin C) may be infused directly into the abdominal cavity after cytoreductive surgery (curgery removing all visible disease to kill remaining microscopic cancerous tumors and free floating cells. The heated chemotherapy (HIPEC) is perfused throughout the abdominal cavity for an hour or two as the last step in the surgery, or ports are installed to allow circulation and/or drainage of the chemicals for one to five days after surgery, known as early postoperative intraperitoneal chemotherapy (EPIC). EPIC may be given in multiple cycles for several months after surgery.[14]
Systemic chemotherapy may be administered as additional or adjuvant treatment. Due to the increased availability of new chemotherapies developed for colorectal cancer patients, some patients have experienced stability in tumor growth with systemic chemotherapy. Systemic chemotherapy is generally reserved for patients with advanced disease, recurrent disease, or disease that has spread to the lymph nodes or distant sites.
This disease may recur following surgery and chemotherapy. Periodic post operative CT scans and tumor marker laboratory tests are used to monitor patients for disease progression.
Epidemiology
The overall incidence was previously estimated at 0.5 to 1 cases per 100,000 people per year.[15] Recent research in Europe indicates that the previous estimate of 1-2 persons per million may be underestimating the actual rate by approximately half, with the real incidence being approximately 3.2 persons per million, and the prevalence being 22 persons per million.[16] It is slightly more common in women than men (male:female ratio of approximately 1:1.3,[17][18]), although the actual ratio is difficult to identify due to potential misdiagnoses and possibly inclusion bias in reported studies. The median age at presentation is typically about 50 years with a range of 20–25 years, but PMP may strike persons of any age,.[19][20]
History
The first case was described by Carl F. Rokitansky in 1842. Werth in 1884 coined the term pseudomyxoma peritonei, describing it in association with a mucinous ovarian tumor. In 1901 Frankel described the first case associated with a cyst of the appendix.
See also
References
- Qu Z, Liu L (2006). "Management of pseudomyxoma peritonei". World J Gastroenterol. 12 (38): 6124–7. doi:10.3748/wjg.v12.i38.6124. PMC 4088104. PMID 17036382.
- Chua; et al. (2012). "Early- and Long-Term Outcome Data of Patients With Pseudomyxoma Peritonei From Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy". Journal of Clinical Oncology. 30 (20): 2449–2456. doi:10.1200/JCO.2011.39.7166. PMID 22614976.
- Young R (2004). "Pseudomyxoma peritonei and selected other aspects of the spread of appendiceal neoplasms". Semin Diagn Pathol. 21 (2): 134–50. doi:10.1053/j.semdp.2004.12.002. PMID 15807473.
- Jacquemin G, Laloux P (2005). "Pseudomyxoma peritonei: review on a cluster of peritoneal mucinous diseases". Acta Chir Belg. 105 (2): 127–33. doi:10.1080/00015458.2005.11679685. PMID 15906901. S2CID 24386163.
- Misdraji, Joseph (2010). "Appendiceal mucinous neoplasms: Controversial issues" (PDF). Arch Pathol Lab Med. Pathology Portal. 134 (6): 864–870. doi:10.1043/1543-2165-134.6.864 (inactive 2021-01-11). PMID 20524864. Archived from the original (PDF) on 2013-03-20. Retrieved 2013-04-21.CS1 maint: DOI inactive as of January 2021 (link)
- Ronnett BM, Zahn CM, Kurman RJ, Sugarbaker PH, Shmookler BM (1995). "Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to 'pseudomyxoma peritonei'". Am J Surg Pathol. 19 (12): 1390–408. doi:10.1097/00000478-199512000-00006. PMID 7503361.
- Bradley, R.F.; Cortina, G.; Geisinger, K.R. (October 2007). "Pseudomyxoma peritonei: Review of the controversy". Current Diagnostic Pathology. 13 (5): 410–416. doi:10.1016/j.cdip.2007.05.013.
- e.g., http://surgpathcriteria.stanford.edu/gitumors/appendix-adenocarcinoma/
- Panarelli, Nicole C; Yantiss, Rhonda K (October 2011). "Mucinous Neoplasms of the Appendix and Peritoneum". Archives of Pathology & Laboratory Medicine. 135 (10): 1261–1268. doi:10.5858/arpa.2011-0034-RA. PMID 21970481.
- Nucci, Marisa R. (3 February 2020). Gynecologic pathology : a volume in the series Foundations in diagnostic pathology (Second ed.). p. 865. ISBN 978-0-323-35909-2.
- Sugarbaker P (2006). "New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome?". Lancet Oncol. 7 (1): 69–76. doi:10.1016/S1470-2045(05)70539-8. PMID 16389186.
- Muhamod F, et al. (2011). "A new standard of care for the management of peritoneal surface malignancy". Curr Oncol. 18 (2): e84–e96. doi:10.3747/co.v18i2.663. PMC 3070715. PMID 21505593.
- Harmon R, Sugarbaker P (2005). "Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer". Int Semin Surg Oncol. 2 (1): 3. doi:10.1186/1477-7800-2-3. PMC 549516. PMID 15701175.
- Culliford AT, Paty PB (2001). "Surgical debulking and intrapertioneal chemotherapy for established peritoneal metastases from colon and appendix cancer". Ann Surg Oncol. 8 (10): 787–95. doi:10.1007/s10434-001-0787-9. PMID 11776492. S2CID 5692764.
- Marmor S, Portschy PR, Tuttle TM, Virnig BA (Apr 2015). "The Rise in Appendiceal Cancer Incidence: 2000–2009". J Gastrointest Surg. 19 (4): 743–50. doi:10.1007/s11605-014-2726-7. PMID 25560182. S2CID 24206562.
- Patrick-Brown, Thale Dawn J. H.; Carr, Norman John; Swanson, David M.; Larsen, Stein; Mohamed, Faheez; Flatmark, Kjersti (2020-06-02). "Estimating the Prevalence of Pseudomyxoma Peritonei in Europe Using a Novel Statistical Method". Annals of Surgical Oncology. 28 (1): 252–257. doi:10.1245/s10434-020-08655-8. ISSN 1534-4681. PMC 7752784. PMID 32488520.
- Kusamura, S.; Baratti, D.; Hutanu, I.; Gavazzi, C.; Morelli, D.; Iusco, D. R.; Grassi, A.; Bonomi, S.; Virzì, S.; Haeusler, E.; Deraco, M. (August 2015). "The role of baseline inflammatory-based scores and serum tumor markers to risk stratify pseudomyxoma peritonei patients treated with cytoreduction (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)". European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 41 (8): 1097–1105. doi:10.1016/j.ejso.2015.04.005. ISSN 1532-2157. PMID 26026742.
- Smeenk, Robert M.; Bruin, Sjoerd C.; van Velthuysen, Marie-Louise F.; Verwaal, Victor J. (August 2008). "Pseudomyxoma peritonei". Current Problems in Surgery. 45 (8): 527–575. doi:10.1067/j.cpsurg.2008.04.003. ISSN 1535-6337. PMID 18590843.
- Fernandes, Ana Claudia de Oliveira; Rocha, Gustavo Ricardo Martins da; Oliveira, Alex Dias de; Guimarães, Marcos Duarte; Carvalho, Stefane Cajango de; Chojniak, Rubens (February 2018). "Pseudomyxoma peritonei in a pediatric patient: A case report and literature review". Revista da Associacao Medica Brasileira (1992). 64 (2): 195–199. doi:10.1590/1806-9282.64.02.195. ISSN 1806-9282. PMID 29641675.
- Sullivan, M. H.; Sugarbaker, P. H. (February 1995). "Treatment of pseudomyxoma peritonei in a geriatric patient population". Journal of Surgical Oncology. 58 (2): 121–124. doi:10.1002/jso.2930580210. ISSN 0022-4790. PMID 7844982. S2CID 19571870.
External links
| Classification | |
|---|---|
| External resources |