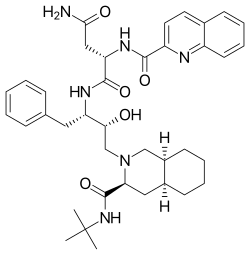
Saquinavir
Saquinavir (SQV), sold under the brand names Invirase and Fortovase, is an antiretroviral drug used together with other medications to treat or prevent HIV/AIDS.[3] Typically it is used with ritonavir or lopinavir/ritonavir to increase its effect.[3] It is taken by mouth.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Invirase, Fortovase |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696001 |
| License data |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~4% (without ritonavir boosting)[2] |
| Protein binding | 98% |
| Metabolism | Liver, mainly by CYP3A4 |
| Elimination half-life | 9–15 hours |
| Excretion | feces (81%) and urine (3%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C38H50N6O5 |
| Molar mass | 670.855 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include nausea, vomiting, diarrhea, and feeling tired.[3] More serious side effects include problems with QT prolongation, heart block, high blood lipids, and liver problems.[3] It appears to be safe in pregnancy.[3] It is in the protease inhibitor class and works by blocking the HIV protease.[3]
Saquinavir was patented in 1988 and first sold in 1995.[4][5]
Medical uses
Saquinavir is used together with other medications to treat or prevent HIV/AIDS.[3] Typically it is used with ritonavir or lopinavir/ritonavir to increase its effect.[3]
Side effects
The most frequent adverse events with saquinavir in either formulation are mild gastrointestinal symptoms, including diarrhoea, nausea, loose stools and abdominal discomfort. Invirase is better tolerated than Fortovase.
Bioavailability and drug interactions
Saquinavir, in the Invirase formulation, has a low and variable oral bioavailability, when given alone. The Fortovase formulation at the standard dosage delivers approximately eightfold more active drug than Invirase, also at the standard dosage.[6]
In the clinic, it was found that the oral bioavailability of saquinavir in both formulations significantly increases when patients also receive the PI ritonavir. For patients, this has the major benefit that they can take less saquinavir, while maintaining sufficient saquinavir blood plasma levels to efficiently suppress the replication of HIV.
The mechanism behind this welcome observation was not directly known, but later it was determined that ritonavir inhibits the cytochrome P450 3A4 isozyme. Normally, this enzyme metabolizes saquinavir to an inactive form, but with the ritonavir inhibiting this enzyme, the saquinavir blood plasma levels increased considerably. Additionally, ritonavir also inhibits multidrug transporters, although to a much lower extent.
Unlike other protease inhibitors, the absorption of saquinavir seems to be improved by omeprazole.[7]
Mechanism of action
Saquinavir is a protease inhibitor. Proteases are enzymes that cleave protein molecules into smaller fragments. HIV protease is vital for both viral replication within the cell and release of mature viral particles from an infected cell. Saquinavir binds to the active site of the viral protease and prevents cleavage of viral polyproteins, preventing maturation of the virus. Saquinavir inhibits both HIV-1 and HIV-2 proteases.
History

Saquinavir was developed by the pharmaceutical company Roche.[9] Saquinavir was the sixth antiretroviral and the first protease inhibitor approved by the US Food and Drug Administration (FDA), leading ritonavir and indinavir by a few months.[10] This new class of antiretrovirals played a critical role in the development of highly active antiretroviral therapy (HAART), which helped significantly lower the risk of death from AIDS-related causes, as seen by a reduction of the annual U.S. HIV-associated death rate, from over 50,000 to about 18,000 over a period of two years.[8][11]
Roche requested and received approval of Invirase via the FDA's "Accelerated Approval" program—a process designed to speed drugs to market for the treatment of serious diseases—a decision that was controversial, as AIDS activists disagreed over the benefits of thorough testing versus early access to new drugs.[12] It was approved again on November 7, 1997, as Fortovase,[13] a soft gel capsule reformulated for improved bioavailability. Roche announced in May 2005 that, given reduced demand, Fortovase would cease being marketed early in 2006, in favor of Invirase boosted with ritonavir,[14] owing to the ability of the latter co-formulated drug to inhibit the enzyme that metabolizes the AIDS drugs.
Society and culture
Economics
As of 2015, it is not available as a generic medication.[15]
Formulations
Two formulations have been marketed:
- a hard-gel capsule formulation of the mesylate, with trade name Invirase, which requires combination with ritonavir to increase the saquinavir bioavailability;
- a soft-gel capsule formulation of saquinavir (microemulsion,[16] orally-administered formulation), with trade name Fortovase, which was discontinued worldwide in 2006.[17]
References
- "Saquinavir Use During Pregnancy". Drugs.com. 20 March 2018. Retrieved 28 January 2020.
- "Invirase- saquinavir mesylate capsule INVIRASE- saquinavir mesylate tablet, film coated". DailyMed. 26 December 2019. Retrieved 28 January 2020.
- "Saquinavir". The American Society of Health-System Pharmacists. Archived from the original on 8 September 2015. Retrieved 5 September 2015.
- Minor, Lisa K. (2006). Handbook of Assay Development in Drug Discovery. Hoboken: CRC Press. p. 117. ISBN 9781420015706. Archived from the original on 31 March 2016.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 509. ISBN 9783527607495.
- "Fortovase". Drugs.com. 22 March 2019. Retrieved 28 January 2020.
- Winston A, Back D, Fletcher C, et al. (2006). "Effect of omeprazole on the pharmacokinetics of saquinavir-500 mg formulation with ritonavir in healthy male and female volunteers". AIDS. 20 (10): 1401–6. doi:10.1097/01.aids.0000233573.41597.8a. PMID 16791014. S2CID 44506039.
- "HIV Surveillance—United States, 1981-2008". Archived from the original on 9 November 2013. Retrieved 8 November 2013.
- J. Hilts, Philip (8 December 1995). "MF.D.A. Backs A New Drug To Fight AIDS". New York Times. Retrieved 28 October 2020.
- "Antiretroviral Drug Discovery and Development". NIH. 26 November 2018. Retrieved 29 October 2020.
- The CDC, in its Morbidity and Mortality Weekly Report, ascribes this to "highly active antiretroviral therapy", without mention of either of these drugs, see the preceding citation. A further citation is needed to make this accurate connection between this drop and the introduction of the protease inhibitors.
- "Drugs! Drugs! Drugs! An Overview of the Approved Anti-HIV Medications". The Body. Archived from the original on 9 November 2013. Retrieved 20 February 2013.
- "Drug Approval Package: Fortovase/Saquinavir NDA 20828". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 28 January 2020.
- Withdrawal of Fortovase (PDF) Archived 2006-05-14 at the Wayback Machine
- "Generic Invirase Availability". Drugs.com. Retrieved 9 July 2020.
- Gibaud S, Attivi D (August 2012). "Microemulsions for oral administration and their therapeutic applications" (PDF). Expert Opinion on Drug Delivery. 9 (8): 937–51. doi:10.1517/17425247.2012.694865. PMID 22663249. S2CID 28468973.
- News-Medical.Net. May 18, 2005 Roche to discontinue the sale and distribution of Fortovase (saquinavir) Archived 2015-02-22 at the Wayback Machine
External links
- "Saquinavir". Drug Information Portal. U.S. National Library of Medicine.
