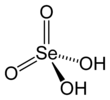
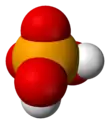
Selenic acid
Selenic acid is the inorganic compound with the formula H
2SeO
4. It is an oxoacid of selenium, and its structure is more accurately described as (HO)
2SeO
2. It is a colorless compound. Although it has few uses, its derivative sodium selenate is used in the production of glass and animal feeds.[2]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Selenic(VI) acid | |||
| Other names
Selenic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.072 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| H 2SeO 4 | |||
| Molar mass | 144.9734 g/mol | ||
| Appearance | Colorless deliquescent crystals | ||
| Density | 2.95 g/cm3, solid | ||
| Melting point | 58 °C (136 °F; 331 K) | ||
| Boiling point | 260 °C (500 °F; 533 K) (decomposes) | ||
| 130 g/100 mL (30 °C) | |||
| Acidity (pKa) | -3, 1.9[1] | ||
| Conjugate base | Hydrogen selenate | ||
| −51.2·10−6 cm3/mol | |||
Refractive index (nD) |
1.5174 (D-line, 20 °C) | ||
| Structure | |||
| tetrahedral at Se | |||
| Hazards | |||
| Main hazards | Corrosive, highly toxic | ||
| R-phrases (outdated) | 23/25-33-50/53 | ||
| S-phrases (outdated) | 20/21-28-45-60-61 | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions |
selenious acid hydrogen selenide | ||
Other cations |
sodium selenate | ||
Related compounds |
Sulfuric acid Selenium dioxide Selenium trioxide Telluric acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure and bonding
The molecule is tetrahedral, as predicted by VSEPR theory. The a Se–O bond length is 161 pm.[3] In the solid state, it crystallizes in an orthorhombic structure.[4]
Preparation
It is prepared by oxidising selenium compounds in lower oxidation states. One method involves the oxidation of selenium dioxide with hydrogen peroxide:
- SeO
2 + H
2O
2 → H
2SeO
4.
Unlike the production sulfuric acid by hydration of sulfur trioxide, the hydration of selenium trioxide is an impractical method.[3] Instead, selenic acid may also be prepared by the oxidation of selenous acid (H
2SeO
3) with halogens, such as chlorine or bromine, or with potassium permanganate.[5] However, using chlorine or bromine as the oxidising agent also produces hydrochloric or hydrobromic acid as a side-product, which needs to be removed from the solution since they can reduce the selenic acid to selenous acid.[6]
Another method of preparing selenic acid is by the oxidation of elemental selenium in a water suspension by chlorine:[5]
- Se + 4 H
2O + 3 Cl
2 → H
2SeO
4 + 6 HCl
To obtain the anhydrous acid as a crystalline solid, the resulting solution is evaporated at temperatures below 140 °C (413 K; 284 °F) in a vacuum.[7]
Reactions
Like sulfuric acid, selenic acid is a strong acid that is hygroscopic and extremely soluble in water. Concentrated solutions are viscous. Crystalline mono- and di-hydrates are known.[5] The monohydrate melts at 26 °C, and the dihydrate melts at −51.7 °C.[3]
Selenic acid is a stronger oxidizer than sulfuric acid,[5] capable of liberating chlorine from chloride ions, being reduced to selenous acid in the process:
- H
2SeO
4 + 2 H+
+ 2 Cl−
→ H
2SeO
3 + H
2O + Cl
2
It decomposes above 200 °C, liberating oxygen gas and being reduced to selenous acid:[5]
- 2 H
2SeO
4 → 2 H
2SeO
3 + O
2
Selenic acid reacts with barium salts to precipitate BaSeO
4, analogous to the sulfate. In general, selenate salts resemble sulfate salts, but are more soluble. Many selenate salts have the same crystal structure as the corresponding sulfate salts.[3]
Treatment with fluorosulfuric acid gives selenoyl fluoride:[7]
- H
2SeO
4 + 2 HO
3SF → SeO
2F
2 + 2 H
2SO
4
Hot, concentrated selenic acid reacts with gold, forming a reddish-yellow solution of gold(III) selenate:[8]
- 2 Au + 6 H
2SeO
4 → Au
2(SeO
4)
3 + 3 H
2SeO
3 + 3 H
2O
Applications
Selenic acid is used as a specialized oxidizing agent.
References
- Magdi Selim, H. (2011-03-15). Dynamics and Bioavailability of Heavy Metals in the Rootzone. ISBN 9781439826232.
- Bernd E. Langner "Selenium and Selenium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a23_525.
- Don M. Yost (2007). Systematic Inorganic Chemistry. Read Books. pp. 343–346. ISBN 978-1-4067-7302-6.
- Mathias S. Wickleder (2007). Francesco A. Devillanova (ed.). Handbook of chalcogen chemistry: new perspectives in sulfur, selenium and tellurium. Royal Society of Chemistry. p. 353. ISBN 978-0-85404-366-8.
- Anil Kumar De (2003). A Text Book of Inorganic Chemistry. New Age International. pp. 543–545. ISBN 81-224-1384-6.
- Lenher, V.; Kao, C. H. (June 1925). "The preparation of selenic acid and of certain selenates". Journal of the American Chemical Society. 47 (6): 1521–1522. doi:10.1021/ja01683a005.
- Seppelt, K. “Selenoyl difluoride” Inorganic Syntheses, 1980, volume XX, pp. 36-38. ISBN 0-471-07715-1. The report describes the synthesis of selenic acid.
- Lenher, V. (April 1902). "Action of selenic acid on gold". Journal of the American Chemical Society. 24 (4): 354–355. doi:10.1021/ja02018a005.