Selenium disulfide
Selenium disulfide, also known as selenium sulfide, is a chemical compound and medication used to treat pityriasis versicolor, seborrhoeic dermatitis, and dandruff.[1] It is applied to the affected area as a lotion or shampoo.[2] Dandruff frequently returns if treatment is stopped.[3]
| Clinical data | |
|---|---|
| Trade names | Selseb, Selsun Blue, others |
| Other names | Selenium sulfide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682258 |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.028.458 |
| Chemical and physical data | |
| Formula | S2Se |
| Molar mass | 143.09 g·mol−1 |
| 3D model (JSmol) | |
| Density | 3 g/cm3 |
| Melting point | 111 °C (232 °F) |
| Boiling point | 118 to 119 °C (244 to 246 °F) (decomposes) |
| Solubility in water | negligible mg/mL (20 °C) |
| |
| |
Side effects include hair loss, irritation of the skin, weakness, and feeling tired.[1] Use is not recommended in children less than 2–5 years old.[1][3] Use in pregnancy or breastfeeding has not been studied.[4] Selenium disulfide is an inorganic compound with the chemical formula SeS2.[5]
Selenium disulfide was approved for medical use in the United States at least as early as 1951.[3] It is on the World Health Organization's List of Essential Medicines.[6] Selenium disulfide is available as a generic medication and over the counter.[2]
Medical uses
Selenium disulfide is sold as an antifungal agent in shampoos for the treatment of dandruff and seborrheic dermatitis associated in the scalp with fungi of genus Malassezia.[7][8][9] In the United States, a 1% strength is available over-the-counter, and a 2.5% strength is also available with a prescription. In Canada, the 2.5% strength is available over-the-counter. At the 2.5% strength, selenium disulfide is also used on the body to treat Tinea versicolor, a type of fungal skin infection caused by a different species of Malassezia. It has been suggested to be effective as a treatment for hyperkeratosis.[10]
Side effects
Selenium disulfide can cause discoloration of the hair and alter the color of hair dyes. It may also discolor metallic jewellery.
Chemical composition
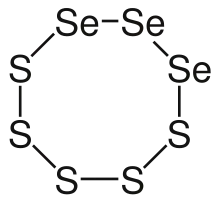
Selenium disulfide has a composition that approximates to SeS2 and is sometimes called selenium sulfide. However, as used in proprietary formulations, it is not a pure chemical compound but a mixture where the overall Se:S ratio is 1:2. The compounds are Se–S rings containing a variable number of S and Se atoms, SenS8−n.[11]
Many selenium sulfides are known as indicated by 77Se-NMR spectroscopy.[12]
History
Selenium monosulfide, along with elemental selenium and sulfur, has been used in medicinal preparations in the past,[13] causing confusion and contradiction[14] as to exactly what form selenium is in any given topical preparation.
Society and culture
In the film Evolution selenium was mentioned as an active ingredient of Head & Shoulders. A group of academics, therefore, tried to use this brand of shampoo to stop an alien invasion after discovering that the alien life form was sensitive to selenium.[15]
See also
- Ketoconazole, another antifungal agent used in medicated shampoos
- Selsun Blue, a shampoo with selenium disulfide as its active ingredient
- Zinc pyrithione, an antimicrobial agent used in many off the shelf shampoos
- Selenium hexasulfide, one possible Se–S ring
References
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 297. hdl:10665/44053. ISBN 9789241547659.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 194. ISBN 9781284057560.
- "Selenium Sulfide". The American Society of Health-System Pharmacists. Archived from the original on 18 January 2017. Retrieved 8 January 2017.
- "Selenium sulfide topical Use During Pregnancy". Drugs.com. Archived from the original on 16 January 2017. Retrieved 13 January 2017.
- Mitchell, Stephen C. (2003). Biological Interactions Of Sulfur Compounds. CRC Press. p. 174. ISBN 9780203362525. Archived from the original on 2017-01-16.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Selenium(IV) sulfide - pharmacy codes search engine Archived 2008-04-01 at the Wayback Machine
- Chemicals of Selenium .Se Archived 2008-04-03 at the Wayback Machine
- Accessed Dec. 24, 2007 Archived 2008-12-26 at the Wayback Machine
- Cohen PR, Anderson CA (December 2018). "Topical Selenium Sulfide for the Treatment of Hyperkeratosis". Dermatology and Therapy. 8 (4): 639–46. doi:10.1007/s13555-018-0259-9. PMC 6261123. PMID 30203232.
- Cyclic selenium sulfides R. Steudel, R. Laitinen, Topics in Current Chemistry, (1982), 102, 177-197
- Pekonen, Pentti.; Hiltunen, Yrjō; Laitinen, Risto S.; Pakkanen, Tapani A. (1991). "Chalcogen ring interconversion pathways. 77Se NMR spectroscopic study of the decomposition of 1,2,3,4,5-Se5S2 to 1,2,3,4,5,6-Se6S2 and 1,2,3,4-Se4S2". Inorganic Chemistry. 30 (19): 3679. doi:10.1021/ic00019a022.
- "Definition: selenium sulfide from Online Medical Dictionary".
- "DrugBank: DB00971 (Selenium Sulfide)". Archived from the original on 2007-04-27.
- "Evolution (2001) - IMDb". Retrieved 19 May 2020.
Further reading
- Danby, FW; Maddin, WS; Margesson, LJ; Rosenthal, D (December 1993). "A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff". Journal of the American Academy of Dermatology. 29 (6): 1008–12. doi:10.1016/0190-9622(93)70282-x. PMID 8245236.
- Grover, R. W. (1956). "Diffuse Hair Loss Associated with Selenium (Selsun) Sulfide Shampoo". JAMA: The Journal of the American Medical Association. 160 (16): 1397–8. doi:10.1001/jama.1956.02960510023006. PMID 13306564.
- Givens, T. G.; Murray, M. M.; Baker, R. C. (1995). "Comparison of 1% and 2.5% Selenium Sulfide in the Treatment of Tinea Capitis". Archives of Pediatrics and Adolescent Medicine. 149 (7): 808–11. doi:10.1001/archpedi.1995.02170200098016. PMID 7795774.
- Ransone, James W.; Scott, Norman M.; Knoblock, Edward C. (1961). "Selenium Sulfide Intoxication". New England Journal of Medicine. 264 (8): 384–5. doi:10.1056/NEJM196102232640806. PMID 13739506.
- Laitinen, Risto S.; Pakkanen, Tapani A. (1987). "77Se NMR spectroscopic characterization of selenium sulfide ring molecules SenS8−n". Inorganic Chemistry. 26 (16): 2598. doi:10.1021/ic00263a010.