Selenium oxydichloride
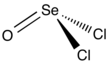
Selenium oxydichloride is the inorganic compound with the formula SeOCl2. It is a colorless liquid. With a high dielectric constant (55) and high specific conductance, it is an attractive solvent. Structurally, it is a close chemical relative of thionyl chloride SOCl2, being a pyramidal molecule.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Selenium oxychloride | |||
| Other names
Seleninyl chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.313 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| SeOCl2 | |||
| Molar mass | 165.87 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 2.43 g/cm3, liquid | ||
| Melting point | 10.9 °C (51.6 °F; 284.0 K) | ||
| Boiling point | 177.2 °C (351.0 °F; 450.3 K) | ||
Refractive index (nD) |
1.651 (20 °C) | ||
| Structure | |||
| trigonal pyramidal | |||
| Hazards | |||
| R-phrases (outdated) | 14-23/25-33-35-50/53 | ||
| S-phrases (outdated) | 26-36/37/39-45-60-61 | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published) |
2 mg/kg (rabbit, dermal)[1] | ||
| Related compounds | |||
Related compounds |
SOCl2, POCl3 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and reactions
Selenium oxydichloride can be prepared by several methods, and a common one involves the conversion of selenium dioxide to dichloroselenious acid followed by dehydration:[2]
- SeO2 + 2 HCl → Se(OH)2Cl2
- Se(OH)2Cl2 → SeOCl2 + H2O
The original synthesis involved the redistribution reaction of selenium dioxide and selenium tetrachloride.
The compound hydrolyzes readily to form hydrogen chloride and selenium dioxide.
See also
- Selenium oxybromide SeOBr2
- Selenous acid H2SeO3
References
- "Selenium compounds (as Se)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Smith, G. B. L.; Jackson, Julius (1950). "Selenium(IV) Oxychloride". Inorganic Syntheses. Inorganic Syntheses. 3. pp. 130–137. doi:10.1002/9780470132340.ch34. ISBN 9780470132340.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.