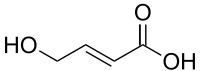
T-HCA
trans-4-Hydroxycrotonic acid (T-HCA), also known as γ-hydroxycrotonic acid (GHC), is an agent used in scientific research to study the GHB receptor.[1] It is an analogue of γ-hydroxybutyric acid (GHB), as well as an active metabolite of GHB.[2][3][4] Similarly to GHB, T-HCA has been found to be endogenous to the rat central nervous system, and as a metabolite of GHB, is almost certain to be endogenous to humans as well.[3][5] T-HCA binds to the high-affinity GHB receptor with 4-fold greater affinity than GHB itself,[6] where it acts as an agonist,[1][7] but does not bind to the low-affinity GHB binding site, the GABAB receptor.[3][8] Because of this, T-HCA does not produce sedation, and instead causes convulsions, which are thought to be caused by GHB receptor activation-evoked increases in extracellular glutamate concentrations, with one notable area where this occurs being the hippocampus.[8]
 | |
| Clinical data | |
|---|---|
| Other names | trans-4-hydroxycrotonic acid |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C4H6O3 |
| Molar mass | 102.089 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
References
- Godfrey Tunnicliff; Christopher D. Cash (2 September 2003). Gamma-Hydroxybutyrate: Pharmacological and Functional Aspects. CRC Press. pp. 24, 104. ISBN 978-0-203-30099-2.
- Quang LS, Desai MC, Kraner JC, Shannon MW, Woolf AD, Maher TJ (2002). "Enzyme and receptor antagonists for preventing toxicity from the gamma-hydroxybutyric acid precursor 1,4-butanediol in CD-1 mice". Ann. N. Y. Acad. Sci. 965 (1): 461–72. Bibcode:2002NYASA.965..461Q. doi:10.1111/j.1749-6632.2002.tb04187.x. PMID 12105121.
- Bourguignon JJ, Schoenfelder A, Schmitt M, Wermuth CG, Hechler V, Charlier B, Maitre M (1988). "Analogues of gamma-hydroxybutyric acid. Synthesis and binding studies". J. Med. Chem. 31 (5): 893–7. doi:10.1021/jm00400a001. PMID 3361576.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 414–. ISBN 978-1-60913-345-0.
- Vayer P, Dessort D, Bourguignon JJ, Wermuth CG, Mandel P, Maitre M (1985). "Natural occurrence of trans-gamma hydroxycrotonic acid in rat brain". Biochem. Pharmacol. 34 (13): 2401–4. doi:10.1016/0006-2952(85)90804-4. PMID 4015683.
- Wellendorph P, Høg S, Greenwood JR, de Lichtenberg A, Nielsen B, Frølund B, Brehm L, Clausen RP, Bräuner-Osborne H (2005). "Novel cyclic gamma-hydroxybutyrate (GHB) analogs with high affinity and stereoselectivity of binding to GHB sites in rat brain". J. Pharmacol. Exp. Ther. 315 (1): 346–51. doi:10.1124/jpet.105.090472. PMID 16014570. S2CID 10332754.
- Encyclopedia of Basic Epilepsy Research. Academic Press. 27 May 2009. pp. 44–. ISBN 978-0-12-373961-2.
- Castelli MP, Ferraro L, Mocci I, Carta F, Carai MA, Antonelli T, Tanganelli S, Cignarella G, Gessa GL (2003). "Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of gamma-hydroxybutyric acid". J. Neurochem. 87 (3): 722–32. doi:10.1046/j.1471-4159.2003.02037.x. PMID 14535954.
