gamma-Amino-beta-hydroxybutyric acid
γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), and sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan, and Mexico.[1][2] It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.[2][3][4][5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gamibetal, others |
| Other names | Buxamine; Buxamina; Bussamina; γ-Amino-β-hydroxybutyric acid; GABOB; β-Hydroxy-γ-aminobutyric acid; β-Hydroxy-GABA |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.916 |
| Chemical and physical data | |
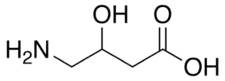
| Formula | C4H9NO3 |
| Molar mass | 119.120 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pharmacology
GABOB is a GABA receptor agonist.[6] It has two stereoisomers, and shows stereoselectivity in its actions.[6] Specifically, (R)-(–)-GABOB is a moderate-potency agonist of the GABAB receptor, while (S)-(+)-GABOB is a partial agonist of the GABAB receptor and an agonist of the GABAA receptor.[6] (S)-(+)-GABOB is around twice as potent an anticonvulsant as (R)-(–)-GABOB.[7] GABOB is used medically as a racemic mixture.[6]
Relative to GABA, GABOB has more potent inhibitory effects on the central nervous system, perhaps due to its greater capacity to cross the blood–brain barrier.[5][8] However, GABOB is of relatively low potency as an anticonvulsant when used by itself, and is more useful as an adjuvant treatment used alongside another anticonvulsant.[9][10]
Chemistry
GABOB, or β-hydroxy-GABA, is a close structural analogue of GABA (see GABA analogue), as well as of γ-hydroxybutyric acid (GHB), phenibut (β-phenyl-GABA), baclofen (β-(4-chlorophenyl)-GABA),[11] and pregabalin (β-isobutyl-GABA).
Society and culture
Generic name
GABOB has been referred to by the generic name buxamine or buxamina.[1][6]
References
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 44–. ISBN 978-3-88763-075-1.
- Michael Bryant Smith (23 October 2013). Methods of Non-α-Amino Acid Synthesis, Second Edition. CRC Press. pp. 146–. ISBN 978-1-4665-7789-3.
- Jack R. Cooper; Floyd E. Bloom; Robert H. Roth (2003). The Biochemical Basis of Neuropharmacology. Oxford University Press. pp. 112–. ISBN 978-0-19-514007-1.
- Melis, Gian B.; Paoletti, A. M.; Mais, V.; Mastrapasqua, N. M.; Strigini, F.; Fruzzetti, F.; Guarnieri, G.; Gambacciani, M.; Fioretti, P. (2014). "Dose-related effects of γ-amino β-hydroxy butyric acid (GABOB) infusion on growth hormone secretion in normal women". Journal of Endocrinological Investigation. 5 (2): 101–106. doi:10.1007/BF03350499. ISSN 0391-4097. PMID 7096918. S2CID 71239193.
- Hayashi, Takashi (1959). "The inhibitory action of β-hydroxy-γ-aminobutyric acid upon the seizure following stimulation of the motor cortex of the dog". The Journal of Physiology. 145 (3): 570–578. doi:10.1113/jphysiol.1959.sp006163. ISSN 0022-3751. PMC 1356963. PMID 13642322.
- Giancarlo Colombo (17 January 2017). GABAB Receptor. Springer. pp. 25–. ISBN 978-3-319-46044-4.
- Roberts E, Krause DN, Wong E, Mori A (1981). "Different Efficacies of d- and l-γ-Amino-β-Hydroxybutyric Acids in GABA Receptor and Transport Test Systems". Journal of Neuroscience. 1 (2): 132–140. doi:10.1523/JNEUROSCI.01-02-00132.1981. PMC 6564147. PMID 6267220.
- De Maio, D.; Pasquariello, G. (1963). "Gamma-amino-beta-hydroxybutyric acid (GABOB) and brain serotonin". Psychopharmacologia. 5 (1): 84–86. doi:10.1007/BF00405577. ISSN 0033-3158. PMID 14085623. S2CID 1436623.
- Chemello R, Giaretta D, Pellegrini A, Testa G (1980). "[Effect of gamma-amino-beta-hydroxybutyric acid (GABHB) on experimentally-induced epileptic activity]" [Effect of γ-amino-β-hydroxybutyric acid (GABHB) on experimentally-induced epileptic activity]. Rivista di Neurologia (in Italian). 50 (4): 253–268. PMID 7466221.
- García-Flores E, Farías R (1997). "γ-Amino-β-hydroxybutyric acid as add-on therapy in adult patients with severe focal epilepsy". Stereotactic and Functional Neurosurgery. 69 (1–4 Pt 2): 243–6. doi:10.1159/000099882. PMID 9711762.
- Lapin I (2001). "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug". CNS Drug Rev. 7 (4): 471–81. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 181–. ISBN 978-3-7692-2114-5.