Triazole
A triazole refers to any of the heterocyclic compounds with molecular formula C2H3N3, having a five-membered ring of two carbon atoms and three nitrogen atoms. There are two sets of isomers that differ in the relative positions of the three nitrogen atoms. Each of these has two tautomers that differ by which nitrogen has a hydrogen bonded to it:
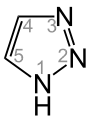 1H-1,2,3-Triazole
1H-1,2,3-Triazole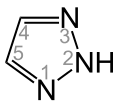 2H-1,2,3-Triazole
2H-1,2,3-Triazole
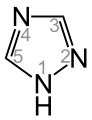 1H-1,2,4-Triazole
1H-1,2,4-Triazole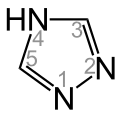 4H-1,2,4-Triazole
4H-1,2,4-Triazole
Derivatives
The triazole antifungal drugs include fluconazole, isavuconazole, itraconazole, voriconazole, pramiconazole, ravuconazole, and posaconazole.
The triazole plant protection fungicides include epoxiconazole, triadimenol, propiconazole, prothioconazole, metconazole, cyproconazole, tebuconazole, flusilazole and paclobutrazol.
Paclobutrazol, uniconazole, flutriafol, and triadimefon are used as plant growth retardants.[1]
Brassinazole is Brassinosteroid Biosynthesis Inhibitor.
Benzotriazole is used in chemical photography as a restrainer and fog suppressant.
Cyclohexylethyltriazol was briefly used as an alternative to Cardiazol (Metrazol) in convulsive shock therapy treatment of mental illnesses during the 1940s.
Importance in agriculture
Due to spreading resistance of plant pathogens towards fungicides of the strobilurin class,[2] control of fungi such as Septoria tritici or Gibberella zeae[3] relies heavily on triazoles. Food, like store bought potatoes, contain retardants such as triazole or tetcyclacis.[4]
Importance in chemical synthesis
The azide alkyne Huisgen cycloaddition is a mild and selective reaction that gives 1,2,3-triazoles as products. The reaction has been widely used in bioorthogonal chemistry and in organic synthesis. Triazoles are relatively stable functional groups and triazole linkages can be used in a variety of applications (for example, replacing the phosphate backbone of DNA.[5])
Related heterocycles
External links
References
- Latimer, Joyce. "Using Plant Growth Regulators on Containerized Herbaceous Perennials". Retrieved 2020-09-19.
- Gisi U, Sierotzki H, Cook A, McCaffery A (2002): Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Management Science 58: 859–867.
- Klix MB, Verreet J-A, Beyer M (2007): Comparison of the declining triazole sensitivity of Gibberella zeae and increased sensitivity achieved by advances in triazole fungicide development. Crop Protection 26:683-690.
- Mantecón, Jorge D. (2009). "Control of potato early blight with triazole fungicide using preventive and curative spraying, or a forecasting system". Ciencia e Investigación Agraria. 36 (2). doi:10.4067/S0718-16202009000200013.
- Hiroyuki Isobe et al. (2008) Triazole-Linked Analogue of Deoxyribonucleic Acid (TLDNA): Design, Synthesis, and Double-Strand Formation with Natural DNA, Org. Lett. 10 (17), pp 3729–3732.