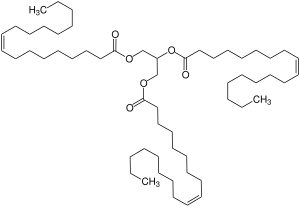
Triolein
Triolein is a symmetrical triglyceride derived from glycerol and three units of the unsaturated fatty acid oleic acid. Most triglycerides are unsymmetrical, being derived from mixtures of fatty acids. Triolein represents 4–30% of olive oil.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Bis[[(Z)-octadec-9-enoyl]oxy]propyl (Z)-octadec-9-enoate; 1,1,1-(propane-1,2,3-triyltrioxy)tris(Z)-octadec-9-enoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.123 |
| MeSH | Triolein |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C57H104O6 | |
| Molar mass | 885.432 g/mol |
| Appearance | Colourless viscous liquid |
| Density | 0.9078 g/cm3 at 25 °C |
| Melting point | 5 °C; 41 °F; 278 K |
| Boiling point | 554.2 °C; 1,029.6 °F; 827.4 K |
| Solubility | Chloroform 0.1g/mL |
| Hazards | |
| Flash point | 302.6 °C (576.7 °F; 575.8 K) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
1.97*105 kJ/kmol |
Gibbs free energy (ΔfG˚) |
-1.8*105 kJ/kmol |
Std enthalpy of combustion (ΔcH⦵298) |
8,389 kcal (35,100 kJ) /mole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triolein is also known as glyceryl trioleate and is one of the two components of Lorenzo's oil.[2]
The oxidation of triolein is according to the formula:
- C
57H
104O
6 + 80 O
2 → 57 CO
2 + 52 H
2O
This gives a respiratory quotient of or 0.7125. The heat of combustion is 8,389 kcal (35,100 kJ) per mole or 9.474 kcal (39.64 kJ) per gram. Per mole of oxygen it is 104.9 kcal (439 kJ).
References
- Alfred Thomas (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173. ISBN 3527306730.
- Lerner, Barron H (2009). "Complicated lessons: Lorenzo Odone and medical miracles". The Lancet. 373 (9667): 888–889. doi:10.1016/S0140-6736(09)60534-1. ISSN 0140-6736.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
