Water cooling
Water cooling is a method of heat removal from components and industrial equipment. Evaporative cooling using water is often more efficient than air cooling. Water is inexpensive and non-toxic however it can contain impurities and cause corrosion.

Water cooling is commonly used for cooling automobile internal combustion engines and power stations. Water coolers utilising convective heat transfer are used inside high-end personal computers to lower the temperature of CPUs.
Other uses include the cooling of lubricant oil in pumps; for cooling purposes in heat exchangers; for cooling buildings in HVAC and in chillers.
Mechanism
Advantages
Water is inexpensive, non-toxic, and available over most of the earth's surface. Liquid cooling offers higher thermal conductivity than air cooling. Water has unusually high specific heat capacity among commonly available liquids at room temperature and atmospheric pressure allowing efficient heat transfer over distance with low rates of mass transfer. Cooling water may be recycled through a recirculating system or used in a single pass once-through cooling (OTC) system. Water's high enthalpy of vaporization allows the option of efficient evaporative cooling to remove waste heat in cooling towers or cooling ponds.[1] Recirculating systems may be open if they rely upon evaporative cooling or closed if heat removal is accomplished in heat exchangers with negligible evaporative loss. A heat exchanger or condenser may separate non-contact cooling water from a fluid being cooled,[2] or contact cooling water may directly impinge on items like saw blades where phase difference allows easy separation. Environmental regulations emphasize the reduced concentrations of waste products in non-contact cooling water.[3]
Disadvantages
Water accelerates corrosion of metal parts and is a favorable medium for biological growth. Dissolved minerals in natural water supplies are concentrated by evaporation to leave deposits called scale. Cooling water often requires addition of chemicals to minimize corrosion and insulating deposits of scale and biofouling.[4]
Water contains varying amounts of impurities from contact with the atmosphere, soil, and containers. Manufactured metals tend to revert to ores via electrochemical reactions of corrosion. Water can accelerate corrosion of machinery being cooled as both an electrical conductor and solvent for metal ions and oxygen. Corrosion reactions proceed more rapidly as temperature increases.[4] Preservation of machinery in the presence of hot water has been improved by addition of corrosion inhibitors including zinc, chromates and phosphates.[5][6] The first two have toxicity concerns;[7] and the last has been associated with eutrophication.[8] Residual concentrations of biocides and corrosion inhibitors are of potential concern for OTC and blowdown from open recirculating cooling water systems.[9] With the exception of machines with short design life, closed recirculating systems require periodic cooling water treatment or replacement raising similar concern about ultimate disposal of cooling water containing chemicals used with environmental safety assumptions of a closed system.
Biofouling occurs because water is a favorable environment for many life forms. Flow characteristics of recirculating cooling water systems encourage colonization by sessile organisms to use the circulating supply of food, oxygen and nutrients.[10] Temperatures may become high enough to support thermophilic populations. Biofouling of heat exchange surfaces can reduce heat transfer rates of the cooling system; and biofouling of cooling towers can alter flow distribution to reduce evaporative cooling rates. Biofouling may also create differential oxygen concentrations increasing corrosion rates. OTC and open recirculating systems are most susceptible to biofouling. Biofouling may be inhibited by temporary habitat modifications. Temperature differences may discourage establishment of thermophilic populations in intermittently operated facilities; and intentional short term temperature spikes may periodically kill less tolerant populations. Biocides have been commonly used to control biofouling where sustained facility operation is required.[11]
Chlorine may be added in the form of hypochlorite to decrease biofouling in cooling water systems, but is later reduced to chloride to minimize toxicity of blowdown or OTC water returned to natural aquatic environments. Hypochlorite is increasingly destructive to wooden cooling towers as pH increases. Chlorinated phenols have been used as biocides or leached from preserved wood in cooling towers. Both hypochlorite and pentachlorophenol have reduced effectiveness at pH values greater than 8.[12] Non-oxidizing biocides may be more difficult to detoxify prior to release of blowdown or OTC water to natural aquatic environments.
Concentrations of polyphosphates or phosphonates with zinc and chromates or similar compounds have been maintained in cooling systems to keep heat exchange surfaces clean so a film of gamma iron oxide and zinc phosphate may inhibit corrosion by passivating anodic and cathodic reaction points.[13] These increase salinity and total dissolved solids, and phosphorus compounds may provide the limiting essential nutrient for algal growth contributing to biofouling of the cooling system or to eutrophication of natural aquatic environments receiving blowdown or OTC water. Chromates reduce biofouling in addition to effective corrosion inhibition in the cooling water system, but residual toxicity in blowdown or OTC water has encouraged reduced chromate concentrations and use of less flexible corrosion inhibitors.[7] Blowdown may also contain chromium leached from cooling towers constructed of wood preserved with chromated copper arsenate.
Total dissolved solids or TDS (sometimes called filtrable residue) is measured as the mass of residue remaining when a measured volume of filtered water is evaporated.[14] Salinity measures water density or conductivity changes caused by dissolved materials.[15] Probability of scale formation increases with increasing total dissolved solids. Solids commonly associated with scale formation are calcium and magnesium carbonate and sulfate. Corrosion rates initially increase with salinity in response to increasing electrical conductivity, but then decrease after reaching a peak as higher levels of salinity decrease dissolved oxygen levels.[4]
Some groundwater contains very little oxygen when pumped from wells, but most natural water supplies include dissolved oxygen. Corrosion increases with increasing oxygen concentrations.[4] Dissolved oxygen approaches saturation levels in cooling towers. Dissolved oxygen is desirable in blowdown or OTC water being returned to natural aquatic environments.
Water ionizes into hydronium (H3O+) cations and hydroxide (OH−) anions. The concentration of ionized hydrogen (as protonated water) in a cooling water system is expressed as pH.[16] Low pH values increase rate of corrosion while high pH values encourage scale formation. Amphoterism is uncommon among metals used in water cooling systems, but aluminum corrosion rates increase with pH values above 9. Galvanic corrosion may be severe in water systems with copper and aluminum components. Acid may be added to cooling water systems to prevent scale formation if the pH decrease will offset increased salinity and dissolved solids.[17]
Steam power stations


Few other cooling applications approach the large volumes of water required to condense low pressure steam at power stations.[19] Many facilities, particularly electric power plants, use millions of gallons of water per day for cooling.[20] Water cooling on this scale may alter natural water environments and create new environments. Thermal pollution of rivers, estuaries and coastal waters is a consideration when siting such plants. Water returned to aquatic environments at temperatures higher than the ambient receiving water modify aquatic habitat by increasing biochemical reaction rates and decreasing oxygen saturation capacity of the habitat. Temperature increases initially favor a population shift from species requiring the high-oxygen concentration of cold water to those enjoying advantages of increased metabolic rates in warm water.[10]
Once-through cooling (OTC) systems may be used on very large rivers or at coastal and estuarine sites. These power stations put the waste heat into the river or coastal water. These OTC systems thus rely upon a good supply of river water or seawater for their cooling needs. Such facilities are built with intake structures designed to pump in large volumes of water at a high rate of flow. These structures tend to also pull in large numbers of fish and other aquatic organisms, which are killed or injured on the intake screens.[21] Large flow rates may immobilize slow-swimming organisms including fish and shrimp on screens protecting the small bore tubes of the heat exchangers from blockage. High temperatures or pump turbulence and shear may kill or disable smaller organisms passing the screens entrained with the cooling water.[22]:Ch. A2 Over 1,200 power plants and manufacturers use OTC systems in the U.S.[23]:4–4 and the intake structures kill billions of fish and other organisms each year.[24] More agile aquatic predators consume organisms impinged on the screens; and warm water predators and scavengers colonize the cooling water discharge to feed on entrained organisms.
The U.S. Clean Water Act requires the Environmental Protection Agency (EPA) to issue regulations on industrial cooling water intake structures.[25] EPA issued final regulations for new facilities in 2001 (amended 2003),[21][26] and for existing facilities in 2014.[27]
Cooling towers

As an alternative to OTC, industrial cooling towers may use recirculated river water, coastal water (seawater), or well water. Large mechanical induced-draft or forced-draft cooling towers in industrial plants continuously circulate cooling water through heat exchangers and other equipment where the water absorbs heat. That heat is then rejected to the atmosphere by the partial evaporation of the water in cooling towers where upflowing air is contacted with the circulating downflow of water. The loss of evaporated water into the air exhausted to the atmosphere is replaced by "make-up" fresh river water or fresh cooling water; but volumes of water lost during evaporative cooling may decrease natural habitat for aquatic organisms. Since the evaporation of pure water is replaced by make-up water containing carbonates and other dissolved salts, a portion of the circulating water is also continuously discarded as "blowdown" water to prevent the excessive build-up of salts in the circulating water; and these blowdown wastes may change the receiving water quality.[28]
Internal combustion engines
The water jacket around an engine is very effective at deadening mechanical noises, which makes the engine quieter.
Open method

An open water cooling system makes use of evaporative cooling, lowering the temperature of the remaining (unevaporated) water. This method was common in early internal combustion engines, until scale buildup was observed from dissolved salts and minerals in the water. Modern open cooling systems continuously waste a fraction of recirculating water as blowdown to remove dissolved solids at concentrations low enough to prevent scale formation. Some open systems use inexpensive tap water, but this requires higher blowdown rates than deionized or distilled water. Purified water systems still require blowdown to remove accumulation of byproducts of chemical treatment to prevent corrosion and biofouling.[29]
Pressurization
Water cooling also has a boiling point temperature of around 100 degrees C at atmospheric pressure. Engines operating at higher temperatures may require a pressurized recycle loop to prevent overheating.[30] Modern automotive cooling systems often operate at 15 psi (103 kPa) to raise the boiling-point of the recycling water coolant and reduce evaporative losses.[31]
Antifreeze
The use of water cooling carries the risk of damage from freezing. Automotive and many other engine cooling applications require the use of a water and antifreeze mixture to lower the freezing point to a temperature unlikely to be experienced. Antifreeze also inhibits corrosion from dissimilar metals and can increase the boiling point, allowing a wider range of water cooling temperatures.[31] Its distinctive odor also alerts operators to cooling system leaks and problems that would go unnoticed in a water-only cooling system. The heated coolant mixture can also be used to warm the air inside the car by means of the heater core.
Other additives
Other less common chemical additives are products to reduce surface tension. These additives are meant to increase the efficiency of automotive cooling systems. Such products are used to enhance the cooling of underperforming or undersized cooling systems or in racing where the weight of a larger cooling system could be a disadvantage.
Power electronics and transmitters
Since approximately 1930 it is common to use water cooling for tubes of powerful transmitters. As these devices uses high operation voltages (around 10 kV), the use of deionized water is required and it has to be carefully controlled. Modern solid-state transmitters can be built so that even high power transmitters do not require water cooling. Water cooling is however also sometimes used for thyristors of HVDC valves, for which also the use of deionized water is required.
Liquid cooling maintenance

Liquid cooling techniques are increasingly being used for the thermal management of electronic components. This type of cooling is a solution to ensure the optimisation of energy efficiency while simultaneously minimising noise and space requirements. Especially useful in supercomputers or Data Centers as maintenance of the racks is quick and easy. After disassembly of the rack, advanced technology quick release couplings eliminate spillage for the safety of operators and protects the integrity of fluids (no impurities in the circuits). These couplings are also capable of being locked (Panel mounted?) to allow blind connection in difficult to access areas. It is important in electronics technology to analyse the connection systems to ensure:
- Non-spill sealing (clean break, flush face couplings)
- Compact and lightweight (materials in special aluminum alloys)
- Operator safety (disconnection without spillage)
- Quick-release couplings sized for optimized flow
- Connection guiding system and compensation of misalignment during connection on rack systems
- Excellent resistance to vibration and corrosion
- Designed to withstand a large number of connections even on refrigerant circuits under residual pressure
Computer usage
Water cooling often adds complexity and cost in comparison to air cooling design by requiring a pump, tubing or piping to transport the water, and a radiator, often with fans, to reject the heat to the atmosphere. Depending on the application, water cooling may create an additional element of risk where leakage from the water coolant recycle loop may corrode or short-circuit sensitive electronic components.
The primary advantage of water cooling for cooling CPU cores in computing equipment is transporting heat away from the source to a secondary cooling surface to allow for large, more optimally designed radiators rather than small, relatively inefficient fins mounted directly on the heat source. Cooling hot computer components with various fluids has been in use since at least the Cray-2 in 1982, using Fluorinert. Through the 1990s, water cooling for home PCs slowly gained recognition among enthusiasts, but it started to become noticeably more prevalent after the introduction of the first Gigahertz-clocked processors in the early 2000s. As of 2018, there are dozens of manufacturers of water cooling components and kits, and many computer manufacturers include preinstalled water cooling solutions for their high-performance systems.
Water cooling can be used to cool many computer components, but usually it is used for the CPU and GPUs. Water cooling usually uses a water block, a water pump, and a water-to-air heat exchanger. By transferring device heat to a separate heat exchanger which can variously be made large and use larger, lower-speed fans, water cooling can allow quieter operation, improved processor speeds (overclocking), or a balance of both. Less commonly, Northbridges, Southbridges, hard disk drives, memory, voltage regulator modules (VRMs), and even power supplies can be water-cooled.[32]
Radiator size may vary: from 40mm dual fan (80mm) to 140 quad fan (560mm) and thickness from 30mm to 80mm. Radiator fans may be mounted on one or both sides.
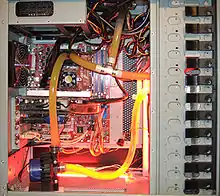
A T-Line is used to remove trapped air bubbles from the circulating water. It is made with a t-connector and a capped-off length of tubing. The tube n acts as a mini-reservoir and allows air-bubbles to travel into it as they are caught into the "tee" connector, and ultimately travel out of the system (bleeding). The capped line may be capped with a fill-port fitting to allow release of trapped gas and addition of liquid.
Water coolers for desktop computers were, until the end of the 1990s, homemade. They were made from car radiators (or more commonly, a car's heater core), aquarium pumps and home-made water blocks, laboratory-grade PVC and silicone tubing and various reservoirs (homemade using plastic bottles, or constructed using cylindrical acrylic or sheets of acrylic, usually clear) and or a T-Line. More recently a growing number of companies are manufacturing water-cooling components compact enough to fit inside a computer case. This, and the trend to CPUs of higher power dissipation, has greatly increased the popularity of water cooling.
Dedicated overclockers occasionally use vapor-compression refrigeration or thermoelectric coolers in place of more common standard heat exchangers. Water cooling systems in which water is cooled directly by the evaporator coil of a phase change system are able to chill the circulating coolant below the ambient air temperature (impossible with a standard heat exchanger) and, as a result, generally provide superior cooling of the computer's heat-generating components. The downside of phase-change or thermoelectric cooling is that it uses much more electricity, and antifreeze must be added due to the low temperature. Additionally, insulation, usually in the form of lagging around water pipes and neoprene pads around the components to be cooled, must be used in order to prevent damage caused by condensation of water vapour from the air on chilled surfaces. Common places from which to borrow the required phase transition systems are a household dehumidifier or air conditioner.[33]
An alternative cooling system, which enables components to be cooled below the ambient temperature, but which obviates the requirement for antifreeze and lagged pipes, is to place a thermoelectric device (commonly referred to as a 'Peltier junction' or 'pelt' after Jean Peltier, who documented the effect) between the heat-generating component and the water block. Because the only sub-ambient temperature zone now is at the interface with the heat-generating component itself, insulation is required only in that localized area. The disadvantage of such a system is a higher power dissipation.
To avoid damage from condensation around the Peltier junction, a proper installation requires it to be "potted" with silicone epoxy. The epoxy is applied around the edges of the device, preventing air from entering or leaving the interior.
Apple's Power Mac G5 was the first mainstream desktop computer to have water cooling as standard (although only on its fastest models). Dell followed suit by shipping their XPS computers with liquid cooling, using thermoelectric cooling to help cool the liquid. Currently, Dell's only computers to offer liquid cooling are their Alienware desktops.[34]
Ships and boats
Water is an ideal cooling medium for vessels as they are constantly surrounded by water that generally remains at a low temperature throughout the year. Systems operating with sea water need to be manufactured from cupronickel, bronze, titanium or similarly corrosion-resistant materials. Water containing sediment may require velocity restrictions through piping to avoid erosion at high velocity or blockage by settling at low velocity.[35]
Other applications
Plant transpiration and animal perspiration use evaporative cooling to prevent high temperatures from causing unsustainable metabolic rates.
Machine guns used in fixed defensive positions sometimes use water cooling to extend barrel life through periods of rapid fire, but the weight of the water and pumping system significantly reduces the portability of water-cooled firearms.
A hospital in Sweden relies on snow-cooling from melt-water from to cool its data centers, medical equipment, and maintain a comfortable ambient temperature.[36]
Some nuclear reactors use heavy water as cooling. Heavy water is employed in nuclear reactors because it is a weaker neutron absorber. This allows for the use of less enriched fuel. For the main cooling system, normal water is preferably employed through the use of a heat exchanger, as heavy water is much more expensive. Reactors that use other materials for moderation (graphite) may also use normal water for cooling.
High-grade industrial water (produced by reverse osmosis or distillation) and potable water are sometimes used in industrial plants requiring high-purity cooling water. Production of these high purity waters creates waste byproduct brines containing the concentrated impurities from the source water.
In 2018, researchers from the University of Colorado Boulder and University of Wyoming invented a radiative cooling metamaterial known as "RadiCold", being developed since 2017. This metamaterial aids in cooling of water and increasing the efficiency of power generation, in which it would cool the underneath objects, by reflecting the sun's rays while at the same time allowing the surface to discharge its heat as an infrared thermal radiation.[37]
See also
- Cooling pond
- Deep lake water cooling
- Free cooling
- Full immersion cooling
- Heat pipe cooling
- Hopper cooling
- Oil cooling
- Peltier cooling
- Thermosiphon (passive heat exchange)
References
- Kemmer (1979), pp. 1-1, 1-2.
- Kemmer (1979), pp. 38-1, 38-4, 38-7 & 38-8.
- King (1995), pp. 143, 439.
- Betz, pp. 183–184.
- Hemmasian-Ettefagh, Ali (2010). "Corrosion Inhibition of Carbon Steel in Cooling Water". Materials Performance. 49: 60–65.
- Mahgoub, F.M.; Abdel-Nabey, B.A.; El-Samadisy, Y.A. (March 2010). "Adopting a multipurpose inhibitor to control corrosion of ferrous alloys in cooling water systems". Materials Chemistry and Physics. 120 (1): 104–108. doi:10.1016/j.matchemphys.2009.10.028. ISSN 0254-0584.
- Kemmer (1979), pp. 38-20, 38-21.
- Goldman & Horne (1983), pp. 153, 160.
- Betz, p. 215.
- Reid (1961), pp. 267–268.
- Betz, p. 202.
- Betz, pp. 203–209.
- Betz, pp. 198–199.
- Franson (1975), pp. 89-98.
- Franson (1975), pp. 99-100.
- Franson (1975), pp. 406-407.
- Betz, pp. 191–194.
- McGeehan, Patrick (2015-05-12). "Fire Prompts Renewed Calls to Close the Indian Point Nuclear Plant". New York Times.
- U.S. Environmental Protection Agency (EPA). (1997). Profile of the Fossil Fuel Electric Power Generation Industry (Report). Washington, D.C. Document No. EPA/310-R-97-007. p. 79.
- EPA (2010). "Partial List of Facilities Subject to Clean Water Act 316(b)."
- EPA (2014). "Cooling Water Intakes."
- Economic and Benefits Analysis for the Final Section 316(b) Phase II Existing Facilities Rule (PDF) (Report). EPA. 2004. EPA 821-R-04-005.
- Technical Development Document for the Final Section 316(b) Existing Facilities Rule (PDF) (Report). EPA. May 2014. EPA 821-R-14-002.
- Final Regulations to Establish Requirements for Cooling Water Intake Structures at Existing Facilities; Fact sheet (PDF) (Report). EPA. May 2014. EPA 821-F-14-001.
- United States. Clean Water Act, Section 316(b), 33 U.S.C. § 1316.
- EPA. Cooling Water Intake Structures. Final rule: 2001-12-18, 66 FR 65255. Amended: 2003-06-19, 68 FR 36749.
- EPA. "National Pollutant Discharge Elimination System—Final Regulations To Establish Requirements for Cooling Water Intake Structures at Existing Facilities and Amend Requirements at Phase I Facilities" Final rule. Federal Register, 79 FR 48300. 2014-08-15.
- Beychok, Milton R. (1967). Aqueous Wastes from Petroleum and Petrochemical Plants (1st ed.). John Wiley and Sons. LCCN 67019834. (See Chapter 2 for material balance relationships in a cooling tower)
- Betz, p. 192.
- Sturgess, Steve (August 2009). "Column: Keep Your Cool". Heavy Duty Trucking. Retrieved April 2, 2018.
- Nice, Karim (2000-11-22). "How Car Cooling Systems Work". HowStuffWorks. HowStuffWorks, Inc. Retrieved 20 August 2012.
- "Koolance 1300/1700W Liquid-Cooled Power Supply". Koolance.com. 2008-03-22. Retrieved 2018-01-19.
- "Dehumidifier & Air Conditioner". extremeoverclocking.com. 2011-04-05. Retrieved 2018-03-11.
- "Alienware Desktops". Dell. Archived from the original on July 28, 2012. Retrieved 2009-11-05.
- Thermex "Heat Exchanger FAQ Page" 2016-12-12.
- "Snow cooling in Sundsvall". www.lvn.se (in Swedish). Retrieved 2017-08-20.
- Dongliang Zhao; Ablimit Aili; Yao Zhai; Jiatao Lu; Dillon Kidd; Gang Tan; Xiaobo Yin; Ronggui Yang (26 October 2018). "Subambient Cooling of Water: Toward Real-World Applications of Daytime Radiative Cooling". Joule. 3: 111–123. doi:10.1016/j.joule.2018.10.006.
Bibliography
- Handbook of Industrial Water Conditioning (7th ed.). Betz Laboratories. 1976.
- Franson, Mary Ann (1975). Standard Methods for the Examination of Water and Wastewater (14th ed.). APHA, AWWA & WPCF. ISBN 0-87553-078-8.
- Goldman, Charles R.; Horne, Alexander J. (1983). Limnology. McGraw-Hill. ISBN 0-07-023651-8.
- Kemmer, Frank N. (1979). The NALCO Water Handbook. McGraw-Hill.
- King, James J. The Environmental Dictionary (3rd Edition). John Wiley & Sons (1995). ISBN 0-471-11995-4
- Reid, George K. Ecology in Inland Waters and Estuaries. Van Nostrand Reinhold (1961).