Aeroplankton
Aeroplankton (or aerial plankton) are tiny lifeforms that float and drift in the air, carried by the current of the wind; they are the atmospheric analogue to oceanic plankton.

| Part of a series on |
| Plankton |
|---|
 |
Most of the living things that make up aeroplankton are very small to microscopic in size, and many can be difficult to identify because of their tiny size. Scientists can collect them for study in traps and sweep nets from aircraft, kites or balloons.[1]
Aeroplankton is made up of numerous microbes, including viruses, about 1000 different species of bacteria, around 40,000 varieties of fungi, and hundreds of species of protists, algae, mosses and liverworts that live some part of their life cycle as aeroplankton, often as spores, pollen, and wind-scattered seeds. Additionally, peripatetic microorganisms are swept into the air from terrestrial dust storms, and an even larger amount of airborne marine microorganisms are propelled high into the atmosphere in sea spray. Aeroplankton deposits hundreds of millions of airborne viruses and tens of millions of bacteria every day on every square meter around the planet.
Overview
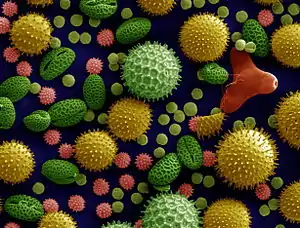
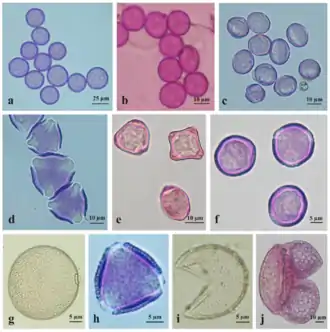
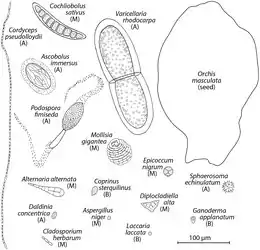
Some cause asthma, such as Alternaria alternata. A drawing of a very small "dust" seed from the flower Orchis maculata is provided for comparison.[3][4]
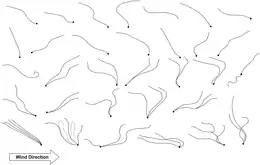
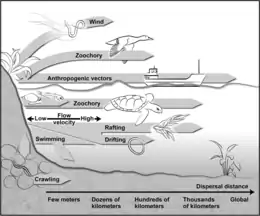
Dispersal is a vital component of an organism’s life-history,[7] and the potential for dispersal determines the distribution, abundance, and thus, the community dynamics of species at different sites.[8][9][10] A new habitat must first be reached before filters such as organismal abilities and adaptations, the quality of a habitat, and the established biocoenosis determine the colonization efficiency of a species.[11] While larger animals can cover distances on their own and actively seek suitable habitats, small (<2 mm) organisms are often passively dispersed,[11] resulting in their more ubiquitous occurrence.[12] While active dispersal accounts for rather predictable distribution patterns, passive dispersal leads to a more randomized immigration of organisms.[8] Mechanisms for passive dispersal are the transport on (epizoochory) or in (endozoochory) larger animals (e.g., flying insects, birds, or mammals) and the erosion by wind.[11][13]
A propagule is any material that functions in propagating an organism to the next stage in its life cycle, such as by dispersal. The propagule is usually distinct in form from the parent organism. Propagules are produced by plants (in the form of seeds or spores), fungi (in the form of spores), and bacteria (for example endospores or microbial cysts).[14] Often cited as important requirement for effective wind dispersal is the presence of propagules (e.g., resting eggs, cysts, ephippia, juvenile and adult resting stages),[11][15][16] which also enables organisms to survive unfavorable environmental conditions until they enter a suitable habitat. These dispersal units can be blown from surfaces such as soil, moss, and the desiccated sediments of temporal waters. The passively dispersed organisms are typically pioneer colonizers.[17][18][19] However, wind-drifted species vary in their vagility (probability to be transported with the wind),[20] with the weight and form of the propagules, and therefore, the wind speed required for their transport,[21] determining the dispersal distance. For example, in nematodes resting eggs are less effectively transported by wind than other life stages,[22] while organisms in anhydrobiosis are lighter and thus more readily transported than hydrated forms.[23][24] Because different organisms are, for the most part, not dispersed over the same distances, source habitats are also important, with the number of organisms contained in air declining with increasing distance from the source system.[17][25] The distances covered by small metazoans range from a few meters,[25] to several hundred meters,[17] and up to several kilometers.[22] While the wind dispersal of aquatic organisms is possible even during the wet phase of a transiently aquatic habitat,[11] during the dry stages a larger number of dormant propagules are exposed to wind and thus dispersed.[16][25][26] Freshwater organisms that must "cross the dry ocean" [11] to enter new aquatic island systems will be passively dispersed more successfully than terrestrial taxa.[11] However, numerous taxa from both soil and freshwater systems have been captured from the air (e.g., bacteria, several algae, ciliates, flagellates, rotifers, crustaceans, mites, and tardigrades).[17][25][26][27] While these have been qualitatively well studied, accurate estimates of their dispersal rates are lacking.[13]
Pollen grains
Recent drilling cores from Switzerland have evidenced the oldest known fossils from flowering plants, pollen grains, which have revealed that flowering plants are 240-million-year-old.[28] Effective pollen dispersal is vital for maintenance of genetic diversity and fundamental for connectivity between spatially separated populations.[29] An efficient transfer of the pollen guarantees successful reproduction in flowering plants. No matter how pollen is dispersed, the male-female recognition is possible by mutual contact of stigma and pollen surfaces. Cytochemical reactions are responsible for pollen binding to a specific stigma.[30][2]
Allergic diseases are considered to be one of the most important contemporary public health problems affecting up to 15–35% of humans worldwide.[31] There is a body of evidence suggesting that allergic reactions induced by pollen are on the increase, particularly in highly industrial countries.[31][32][2]
Fungal spores
A wealth of correlative evidence suggests asthma is associated with fungi and triggered by elevated numbers of fungal spores in the environment.[33] Intriguing are reports of thunderstorm asthma. In a now classic study from the United Kingdom, an outbreak of acute asthma was linked to increases in Didymella exitialis ascospores and Sporobolomyces basidiospores associated with a severe weather event.[34] Thunderstorms are associated with spore plumes: when spore concentrations increase dramatically over a short period of time, for example from 20,000 spores/m3 to over 170,000 spores/m3 in 2 hours.[35] However, other sources consider pollen or pollution as causes of thunderstorm asthma.[36] Transoceanic and transcontinental dust events move large numbers of spores across vast distances and have the potential to impact public health,[37] and similar correlative evidence links dust blown off the Sahara with pediatric emergency room admissions on the island of Trinidad.[38][3]
The study of fungal spores in aeroplankton is called aeromycology.
Fern spores
Pteridophyte spores, such as fern spores, similarly to pollen grains and fungal spores, are also components of aeroplankton.[39][40] Fungal spores usually rank first among bioaerosol constituents due to their highest concentrations (1000–10 000 m−3), while pollen grains and fern spores can reach a similar content (10–100 m−3).[41][32]
Arthropods
Many small animals, mainly arthropods (such as insects and spiders), are also carried upwards into the atmosphere by air currents and may be found floating several thousand feet up. Aphids, for example, are frequently found at high altitudes.
Ballooning, sometimes called kiting, is a process by which spiders, and some other small invertebrates, move through the air by releasing one or more gossamer threads to catch the wind, causing them to become airborne at the mercy of air currents.[42][43] A spider (usually limited to individuals of a small species), or spiderling after hatching,[44] will climb as high as it can, stand on raised legs with its abdomen pointed upwards ("tiptoeing"),[45] and then release several silk threads from its spinnerets into the air. These automatically form a triangular shaped parachute[46] which carries the spider away on updrafts of winds where even the slightest of breezes will disperse the arachnid.[45][46] The flexibility of their silk draglines can aid the aerodynamics of their flight, causing the spiders to drift an unpredictable and sometimes long distance.[47] Even atmospheric samples collected from balloons at five kilometres altitude and ships mid-ocean have reported spider landings. Mortality is high.[48]
The Earth's static electric field may also provide lift in windless conditions.[49]
Nematodes
Nematodes (roundworms), the most common animal taxon, are also found among aeroplankton.[16][17][25] Nematodes are an essential trophic link between unicellular organisms like bacteria, and larger organisms such as tardigrades, copepods, flatworms, and fishes.[13] For nematodes, anhydrobiosis is a widespread strategy allowing them to survive unfavorable conditions for months and even years.[50][51] Accordingly, nematodes can be readily dispersed by wind. However, as reported by Vanschoenwinkel et al.,[25] nematodes account for only about one percent of wind-drifted animals. Among the habitats colonized by nematodes are those that are strongly exposed to wind erosion as e.g., the shorelines of permanent waters, soils, mosses, dead wood, and tree bark.[52][13] In addition, within a few days of forming temporary waters such as phytotelmata were shown to be colonized by numerous nematode species.[19][53][13]
Marine microorganisms
A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes.[54] Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet.[55][56]
Gallery
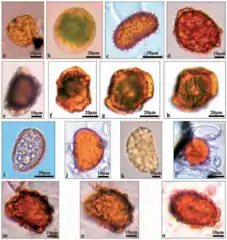 Pteridophyta spores, including fern spores, in the air of Lublin
Pteridophyta spores, including fern spores, in the air of Lublin Airborne fungal spores
Airborne fungal spores.JPG.webp) Detail of the formation of a dense aeroplankton cloud at sunset, over the Loire river in France
Detail of the formation of a dense aeroplankton cloud at sunset, over the Loire river in France
References
- A. C. Hardy and P. S. Milne (1938) Studies in the Distribution of Insects by Aerial Currents. Journal of Animal Ecology, 7(2):199-229
- Denisow, B. and Weryszko-Chmielewska, E. (2015) "Pollen grains as airborne allergenic particles". Acta Agrobotanica, 68(4). doi:10.5586/aa.2015.045.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Pringle, A. (2013) "Asthma and the diversity of fungal spores in air". PLoS Pathogens, 9(6): e1003371. doi:10.1371/journal.ppat.1003371.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Figure adapted from: Ingold CT (1971) Fungal spores: their liberation and dispersal, Oxford: Clarendon Press.
- Cho, M., Neubauer, P., Fahrenson, C. and Rechenberg, I. (2018) "An observational study of ballooning in large spiders: Nanoscale multifibers enable large spiders’ soaring flight". PLoS biology, 16(6): e2004405. doi:10.1371/journal.pbio.2004405.g007.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Ptatscheck, Christoph; Traunspurger, Walter (2020). "The ability to get everywhere: Dispersal modes of free-living, aquatic nematodes". Hydrobiologia. 847 (17): 3519–3547. doi:10.1007/s10750-020-04373-0. S2CID 221110776.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Bonte, Dries; Dahirel, Maxime (2017). "Dispersal: A central and independent trait in life history". Oikos. 126 (4): 472–479. doi:10.1111/oik.03801.
- Rundle, Simon D.; Robertson, Anne L.; Schmid-Araya, Jenny M. (2002). Freshwater Meiofauna: Biology and Ecology. ISBN 9789057821097.
- Kneitel, Jamie M.; Miller, Thomas E. (2003). "Dispersal Rates Affect Species Composition in Metacommunities of Sarracenia purpurea Inquilines". The American Naturalist. 162 (2): 165–171. doi:10.1086/376585. PMID 12858261. S2CID 17576931.
- Cottenie, Karl (2005). "Integrating environmental and spatial processes in ecological community dynamics". Ecology Letters. 8 (11): 1175–1182. doi:10.1111/j.1461-0248.2005.00820.x. PMID 21352441.
- Incagnone, Giulia; Marrone, Federico; Barone, Rossella; Robba, Lavinia; Naselli-Flores, Luigi (2015). "How do freshwater organisms cross the "dry ocean"? A review on passive dispersal and colonization processes with a special focus on temporary ponds". Hydrobiologia. 750: 103–123. doi:10.1007/s10750-014-2110-3. hdl:10447/101976. S2CID 13892871.
- Finlay, B. J. (2002). "Global Dispersal of Free-Living Microbial Eukaryote Species". Science. 296 (5570): 1061–1063. Bibcode:2002Sci...296.1061F. doi:10.1126/science.1070710. PMID 12004115. S2CID 19508548.
- Ptatscheck, Christoph; Gansfort, Birgit; Traunspurger, Walter (2018). "The extent of wind-mediated dispersal of small metazoans, focusing nematodes". Scientific Reports. 8 (1): 6814. Bibcode:2018NatSR...8.6814P. doi:10.1038/s41598-018-24747-8. PMC 5931521. PMID 29717144.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - T.Y. Chuang and W.H. Ko. 1981. Propagule size: Its relation to population density of microorganisms in soil. Soil Biology and Biochemistry. 13(3).
- Panov, Vadim E.; Krylov, Piotr I.; Riccardi, Nicoletta (2004). "Role of diapause in dispersal and invasion success by aquatic invertebrates". Journal of Limnology. 63: 56. doi:10.4081/jlimnol.2004.s1.56.
- Nkem, Johnson N.; Wall, Diana H.; Virginia, Ross A.; Barrett, John E.; Broos, Emma J.; Porazinska, Dorota L.; Adams, Byron J. (2006). "Wind dispersal of soil invertebrates in the Mc Murdo Dry Valleys, Antarctica". Polar Biology. 29 (4): 346–352. doi:10.1007/s00300-005-0061-x. S2CID 32516212.
- Maguire, Bassett (1963). "The Passive Dispersal of Small Aquatic Organisms and Their Colonization of Isolated Bodies of Water". Ecological Monographs. 33 (2): 161–185. doi:10.2307/1948560. JSTOR 1948560.
- Ptatscheck, Chistoph; Traunspurger, Walter (2014). "The meiofauna of artificial water-filled tree holes: Colonization and bottom-up effects". Aquatic Ecology. 48 (3): 285–295. doi:10.1007/s10452-014-9483-2. S2CID 15256569.
- Cáceres, Carla E.; Soluk, Daniel A. (2002). "Blowing in the wind: A field test of overland dispersal and colonization by aquatic invertebrates". Oecologia. 131 (3): 402–408. Bibcode:2002Oecol.131..402C. doi:10.1007/s00442-002-0897-5. PMID 28547712. S2CID 9941895.
- Jenkins, David G. (1995). "Dispersal-limited zooplankton distribution and community composition in new ponds". Hydrobiologia. 313–314: 15–20. doi:10.1007/BF00025926. S2CID 45667054.
- Parekh, Priya A.; Paetkau, Mark J.; Gosselin, Louis A. (2014). "Historical frequency of wind dispersal events and role of topography in the dispersal of anostracan cysts in a semi-arid environment". Hydrobiologia. 740: 51–59. doi:10.1007/s10750-014-1936-z. S2CID 18458173.
- Carroll, J.J. and Viglierchio, D.R. (1981). "On the transport of nematodes by the wind". Journal of Nematology, 13(4): 476.
- Van Gundy, Seymour D. (1965). "Factors in Survival of Nematodes". Annual Review of Phytopathology. 3: 43–68. doi:10.1146/annurev.py.03.090165.000355.
- Ricci, C.; Caprioli, M. (2005). "Anhydrobiosis in Bdelloid Species, Populations and Individuals". Integrative and Comparative Biology. 45 (5): 759–763. doi:10.1093/icb/45.5.759. PMID 21676827. S2CID 42270008.
- Vanschoenwinkel, Bram; Gielen, Saïdja; Seaman, Maitland; Brendonck, Luc (2008). "Any way the wind blows - frequent wind dispersal drives species sorting in ephemeral aquatic communities". Oikos. 117: 125–134. doi:10.1111/j.2007.0030-1299.16349.x.
- Vanschoenwinkel, Bram; Gielen, Saïdja; Vandewaerde, Hanne; Seaman, Maitland; Brendonck, Luc (2008). "Relative importance of different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity". Ecography. 31 (5): 567–577. doi:10.1111/j.0906-7590.2008.05442.x.
- "X. The distribution of micro-organisms in air". Proceedings of the Royal Society of London. 40 (242–245): 509–526. 1886. doi:10.1098/rspl.1886.0077. S2CID 129825037.
- Feist-Burkhardt, Susanne; Hochuli, Peter A. (2013). "Angiosperm-like pollen and Afropollis from the Middle Triassic (Anisian) of the Germanic Basin (Northern Switzerland)". Frontiers in Plant Science. 4: 344. doi:10.3389/fpls.2013.00344. PMC 3788615. PMID 24106492.
- Fénart, Stéphane; Austerlitz, Frédéric; Cuguen, Joël; Arnaud, Jean-François (2007). "Long distance pollen-mediated gene flow at a landscape level: The weed beet as a case study". Molecular Ecology. 16 (18): 3801–3813. doi:10.1111/j.1365-294X.2007.03448.x. PMID 17850547. S2CID 6382777.
- Edlund, A. F.; Swanson, R.; Preuss, D. (2004). "Pollen and Stigma Structure and Function: The Role of Diversity in Pollination". The Plant Cell Online. 16: S84–S97. doi:10.1105/tpc.015800. PMC 2643401. PMID 15075396.
- World Allergy Organization (WAO) White Book on Allergy. 2011. ISBN 9780615461823.
- Cecchi, Lorenzo (2013). "Introduction". Allergenic Pollen. pp. 1–7. doi:10.1007/978-94-007-4881-1_1. ISBN 978-94-007-4880-4.
- Denning, D.W., O'driscoll, B.R., Hogaboam, C.M., Bowyer, P. and Niven, R.M. (2006) "The link between fungi and severe asthma: a summary of the evidence". European Respiratory Journal, 27(3): 615–626. doi:10.1183/09031936.06.00074705.
- Packe, G.E. and Ayres, J. (1985) "Asthma outbreak during a thunderstorm". The Lancet, 326(8448): 199–204. doi:10.1016/S0140-6736(85)91510-7.
- Burch, M. and Levetin, E., 2002. Effects of meteorological conditions on spore plumes. International journal of biometeorology, 46(3), pp.107–117. doi:10.1007/s00484-002-0127-1.
- Bernstein, J.A., Alexis, N., Barnes, C., Bernstein, I.L., Nel, A., Peden, D., Diaz-Sanchez, D., Tarlo, S.M. and Williams, P.B., 2004. Health effects of air pollution. Journal of allergy and clinical immunology, 114(5), pp.1116-1123. doi:10.1016/j.jaci.2004.08.030.
- Kellogg CA, Griffin DW (2006) "Aerobiology and the global transport of desert dust". Trends Ecol Evol, 21: 638–644. doi:10.1016/j.tree.2006.07.004.
- Gyan, K., Henry, W., Lacaille, S., Laloo, A., Lamsee-Ebanks, C., McKay, S., Antoine, R.M. and Monteil, M.A. (2005) "African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad". International Journal of Biometeorology, 49(6): 371–376. doi:10.1007/s00484-005-0257-3.
- Burge, Harriet A.; Rogers, Christine A. (2000). "Outdoor Allergens". Environmental Health Perspectives. 108: 653–659. doi:10.2307/3454401. JSTOR 3454401. PMC 1637672. PMID 10931783. S2CID 16407560.
- Weryszko-Chmielewska, E. (2007). "Zakres badań i znaczenie aerobiologii". Aerobiologia. Lublin: Wydawnictwo Akademii Rolniczej, pages 6-10 (in Polish).
- Després, Vivianer.; Huffman, J.Alex; Burrows, Susannah M.; Hoose, Corinna; Safatov, Aleksandrs.; Buryak, Galina; Fröhlich-Nowoisky, Janine; Elbert, Wolfgang; Andreae, Meinrato.; Pöschl, Ulrich; Jaenicke, Ruprecht (2012). "Primary biological aerosol particles in the atmosphere: A review". Tellus B: Chemical and Physical Meteorology. 64 (1): 15598. Bibcode:2012TellB..6415598D. doi:10.3402/tellusb.v64i0.15598. S2CID 98741728.
- Heinrichs, Ann (2004). Spiders. Compass Point Books. ISBN 9780756505905. OCLC 54027960.
- Valerio, C.E. (1977). "Population structure in the spider Achaearranea Tepidariorum (Aranae, Theridiidae)". Journal of Arachnology. 3 (3): 185–190. JSTOR 3704941.
- Bond, Jason Edward (22 September 1999). Systematics and Evolution of the Californian Trapdoor Spider Genus Aptostichus Simon (Araneae: Mygalomorphae: Euctenizidae) (Thesis). CiteSeerX 10.1.1.691.8754. hdl:10919/29114.
- Weyman, G.S. (1995). "Laboratory studies of the factors stimulating ballooning behavior by Linyphiid spiders (Araneae, Linyphiidae)". Journal of Arachnology. 23 (2): 75–84. JSTOR 3705494.
- Schneider, J.M.; Roos, J.; Lubin, Y.; Henschel, J.R. (October 2001). "Dispersal of Stegodyphus Dumicola (Araneae, Eresidae): They do balloon after all!". Journal of Arachnology. 29 (1): 114–116. doi:10.1636/0161-8202(2001)029[0114:DOSDAE]2.0.CO;2.
- "Leap forward for 'flying' spiders". BBC News. 12 July 2006. Retrieved 23 July 2014.
- Morley, Erica L.; Robert, Daniel (July 2018). "Electric Fields Elicit Ballooning in Spiders". Current Biology. 28 (14): 2324–2330.e2. doi:10.1016/j.cub.2018.05.057. PMC 6065530. PMID 29983315.
- Gorham, Peter (Sep 2013). "Ballooning spiders: The case for electrostatic flight". arXiv:1309.4731 [physics.bio-ph].
- Hendriksen, N. B. (1982) "Anhydrobiosis in nematodes: studies on Plectus sp." In: New trends in soil biology (Eds: Lebrun, P. André, H. M., De Medts, A., Grégoire-Wibo, C. Wauthy, G.) pages 387–394, Louvain-la-Neurve, Belgium.
- Watanabe, M. (2006). "Anhydrobiosis in invertebrates". Applied Entomology and Zoology, 41(1): 15–31. doi:10.1303/aez.2006.15.
- Andrássy, I. (2009) "Free-living nematodes of Hungary III (Nematoda errantia)". Pedozoologica Hungarica No. 5. Hungarian Natural History Museum and Systematic Zoology, Research Group of the Hungarian Academy of Sciences.
- Ptatscheck, Christoph; Dümmer, Birgit; Traunspurger, Walter (2015). "Nematode colonisation of artificial water-filled tree holes". Nematology. 17 (8): 911–921. doi:10.1163/15685411-00002913.
- Living Bacteria Are Riding Earth’s Air Currents Smithsonian Magazine, 11 January 2016.
- Robbins, Jim (13 April 2018). "Trillions Upon Trillions of Viruses Fall From the Sky Each Day". The New York Times. Retrieved 14 April 2018.
- Reche, Isabel; D’Orta, Gaetano; Mladenov, Natalie; Winget, Danielle M; Suttle, Curtis A (29 January 2018). "Deposition rates of viruses and bacteria above the atmospheric boundary layer". ISME Journal. 12 (4): 1154–1162. doi:10.1038/s41396-017-0042-4. PMC 5864199. PMID 29379178.
