Norwood procedure
The Norwood procedure is the first surgery of three staged heart surgeries to create a new functional systemic circuit in patients with hypoplastic left heart syndrome or other complex heart defects with single ventricle physiology. The Norwood procedure (stage 1) involves atrial septectomy and transection and ligation of the distal main pulmonary artery. The proximal pulmonary artery is then connected to the hypoplastic aortic arch, while the coarcted segment of the aorta is repaired. An aortopulmonary shunt is created to connect the aorta to the main pulmonary artery to provide pulmonary blood flow. The second surgery (Stage 2) is the separation of the systemic and pulmonary circulation once pulmonary vascular resistance has fallen, by removing the aortopulmonary shunt followed by the creation of a bidirectional SVC-pulmonary shunt, also known as a modified Glenn procedure or Hemi-Fontan.[1] The third surgery (Stage 3) is the Fontan procedure, in which the inferior vena cava (IVC, the large vein carrying blood back to the heart from the lower part of the body) is connected to the branch pulmonary arteries. After this surgery is completed, all the venous blood returning from the body flows directly to the lungs.
| Norwood procedure | |
|---|---|
 Diagram of a healthy heart and one suffering from Hypoplastic left heart syndrome. In the heart on the right, note the near absence of the left ventricle, which normally provides systemic circulation. Following the three stage palliation (Norwood, Glenn or hemi-Fontan, then Fontan), blood flow from the right ventricle is rerouted to serve this function, which means that an alternative source of pulmonary circulation must be provided. | |
| ICD-9-CM | 35.8 |
The first successful use of the Norwood procedure was reported by Dr. William Imon Norwood, Jr. and colleagues in 1981.[2][3]Cardiopulmonary bypass is required.[4]
Indications
This procedure is most often performed to treat hypoplastic left heart syndrome, certain types of mitral atresia, or other conditions that result in single ventricle circulation.
In these conditions, the most urgent problem is that the heart is unable to pump blood to the systemic circulation (i.e. to the body). The goal of these three surgeries is to ultimately connect the single ventricle to the systemic circulation. To accomplish this, blood flow to the lungs is disrupted, and therefore an alternative path must be created to supply the lungs.
Process
Entry to the body cavity for the Norwood procedure is gained by a vertical incision above the sternum. Separation of the sternum is necessary. This surgery is complex and may vary slightly depending on the diagnosis and overall condition of the heart.The surgery on the heart can be divided into two main steps.[5]
Providing systemic circulation
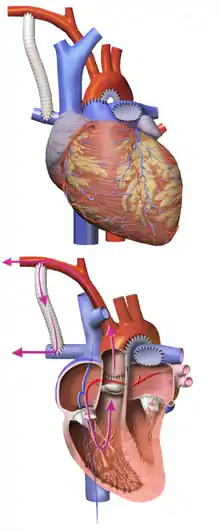
The main pulmonary artery is separated from the left and right portions of the pulmonary artery and joined with the upper portion of the aorta. Widening of the pulmonary artery is often necessary, and may be accomplished by using the patient's existing biological tissue, or appropriate animal tissue. This allows the blood, a mixture of oxygenated and deoxygenated, to be pumped to the body via the morphologic right ventricle, through the pulmonary valve.
Providing pulmonary circulation
Since the remainder of the pulmonary artery is now disconnected from the heart, one of a few techniques must be used to supply blood to the lungs:
- With a modified Blalock-Taussig Shunt, a Gore-Tex conduit (a kind of plastic tubing) is used to connect the subclavian artery to the pulmonary artery. In this case, blood comes from the single ventricle, through the pulmonary valve, the reconstructed aorta, the subclavian artery, and the conduit, to the lungs. There are variations on this procedure where the origin of the shunt is elsewhere in the systemic circulation (e.g. from the aorta itself) rather than the subclavian artery.
- With a Sano shunt, a hole is made in the wall of the single ventricle, and a Gore-Tex conduit is used to connect the ventricle to the pulmonary artery. The key difference here is that the blood flow is more pulsatile than with the Blalock-Taussig version.
After this first step (switching the right ventricle in functional position of the absent left ventricle) children generally proceed down the path to a Fontan procedure.
References
- Gregory's Pediatric Anesthesia Textbook, page 622
- Norwood, WI; Lang, P; Casteneda, AR; Campbell, DN (October 1981). "Experience with operations for hypoplastic left heart syndrome". The Journal of Thoracic and Cardiovascular Surgery. 82 (4): 511–9. doi:10.1016/s0022-5223(19)39288-8. PMID 6168869.
- Norwood, William I.; Lang, Peter; Hansen, Dolly D. (6 January 1983). "Physiologic Repair of Aortic Atresia–Hypoplastic Left Heart Syndrome". New England Journal of Medicine. 308 (1): 23–26. doi:10.1056/NEJM198301063080106. PMID 6847920.
- Ricardo Munoz; Victor Morell; Peter Wearden (August 2009). Critical Care of Children with Heart Disease: Basic Medical and Surgical Concepts. Springer. pp. 326–. ISBN 978-1-84882-261-0. Retrieved 21 June 2011.
- A. Corno; Gigi P. Festa (8 December 2008). Congenital Heart Defects. Decision Making for Surgery: CT-Scan and Clinical Correlations. Springer. pp. 123–. ISBN 978-3-7985-1718-9. Retrieved 24 June 2011.