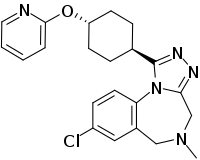
Balovaptan
Balovaptan (INN; developmental code name RG7314), is a selective small molecule antagonist of the vasopressin V1A receptor which is under development by Roche for the treatment of autism.[1] As of August 2019, it is in a phase III clinical trial for adults and a phase II clinical trial for children for this indication.[2] On 29 January 2018, Roche announced that the US Food and Drug Administration (FDA) had granted Breakthrough Therapy Designation for balovaptan in individuals with autism spectrum disorder (ASD).[3] The FDA granted this based on the results of the adult phase II clinical trial called VANILLA (Vasopressin ANtagonist to Improve sociaL communication in Autism) study.[4] The currently-recruiting (Until March 2020) phase III adult study is called V1aduct and the currently-closed (August 2019) phase II child study is called Av1ation.
 | |
| Clinical data | |
|---|---|
| Other names | RG7314 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H24ClN5O |
| Molar mass | 409.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- "Roche - Pipeline". 2014. Retrieved 1 August 2014.
- Clinical trial number NCT01793441 for "Study of RG7314 to Investigate Efficacy and Safety in Individuals With Autism Spectrum Disorders" at ClinicalTrials.gov
- "FDA grants Breakthrough Therapy Designation for Roche's balovaptan in autism spectrum disorder" (Press release). 29 January 2018. Retrieved 6 February 2018.
- Bolognani F, Del Valle Rubido M, Squassante L, Wandel C, Derks M, Murtagh L, et al. (May 2019). "A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder". Science Translational Medicine. 11 (491). doi:10.1126/scitranslmed.aat7838. PMID 31043521.
