Ceritinib
Ceritinib (INN,[2] trade name Zykadia /zaɪˈkeɪdiːə/ zy-KAY-dee-ə) is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC).[3] It was developed by Novartis and received FDA approval for use in April 2014.[3]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /səˈrɪtɪnɪb/ sə-RIT-i-nib |
| Trade names | Zykadia |
| Other names | LDK378 |
| AHFS/Drugs.com | Multum Consumer Information |
| License data | |
| Routes of administration | By mouth (capsules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not determined |
| Protein binding | 97% |
| Metabolism | CYP3A |
| Elimination half-life | 41 hours |
| Excretion | Feces (92.3%), urine (1.3%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.241.919 |
| Chemical and physical data | |
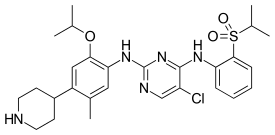
| Formula | C28H36ClN5O3S |
| Molar mass | 558.14 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medical uses
Ceritinib is an anaplastic lymphoma kinase (ALK)-positive inhibitor primarily used for the treatment of metastatic NSCLC.[4] Previously, it was only indicated for patients who had developed resistant to crizotinib, another ALK-positive inhibitor, but has since had its usage expanded to serve as a primary option for metastatic NSCLC.[5]
Pharmacology
Mechanism of action
Ceritinib is a selective and potent inhibitor of anaplastic lymphoma kinase (ALK). In normal physiology, ALK functions as a key step in the development and function of nervous system tissue. However, chromosomal translocation and fusion give rise to an oncogenic form of ALK that has been implicated in progression of NSCLC. Ceritinib thus acts to inhibit this mutated enzyme and stop cell proliferation, ultimately halting cancer progression.[6] Because ceritinib is considered a targeted cancer therapy, an FDA-approved test is required to determine which patients are candidates for ceritinib. This test, developed by Roche, is the VENTANA ALK (D5F3) CDx Assay and is used to identify ALK-positive NSCLC patients who would benefit from ceritinib treatment.[7]
Adverse effects
Serious adverse effects include gastrointestinal toxicity, hepatotoxicity, interstitial lung disease, prolonged QT syndrome, hyperglycemia, bradycardia, and pancreatitis.[8][9]
The most commonly reported side effects were diarrhea, nausea, elevated liver enzymes, vomiting, abdominal pain, fatigue, decreased appetite, and constipation.[4] Due to the risk of elevated liver enzymes, liver function tests should be performed every two weeks for the first 9 weeks of treatment.[10]
Finally, ceritinib is both a substrate and potent inhibitor of the enzyme CYP3A4, so medications should be monitored carefully that may interact with ceritinib.[9]
Dose
Ceritinib is available as a 150 mg capsule with a one-time daily dosing requirement of 450 mg with food.[11]
Research and development
Researchers first identified the ALK fusion gene in 1994. Several years later, Novartis Pharmaceuticals Corporation, began working towards development of targeted ALK inhibitors. In April 2014, the FDA granted accelerated approval for ceritinib when used for ALK-positive NSCLC patients who have progressed on or are intolerant to crizotinib (Xalkori, Pfizer, Inc.). This rapid approval was determined from a multi-center clinical trial in which 163 patients who had disease progression or were intolerant to crizotinib received oral ceritinib 750 mg once daily. This trial demonstrated an objective response rate (ORR) of 44% and a median duration of response (DOR) of 7.1 months, both of which were favorable compared to the worsening or failed use of crizotinib.[12]
In February 2017, the FDA accepted a supplement New Drug Application for ceritinib and granted Priority Review for expanded use of ceritinib. Specifically, it became a first-line therapy option for metastatic NSCLC with ALK-positive tumors. Additionally, the FDA also gave Breakthrough Therapy designation to the drug for ALK-positive metastatic NSCLC that has metastasized to the brain.[13] This new designation resulted from the ASCEND-4 clinical trial, which was a randomized, phase III study that compared the use of ceritinib to standard-of-care platinum-based chemotherapy treatments. Median progression-free survival was 16.6 months for ceritinib (n=189) versus 8.1 months in the chemotherapy-treated patients (n=187).[14]
There are currently no generic options available for ceritinib.
Commercialization
Zykadia is manufactured by Novartis.[15] Created in 1996 from a merger between Ciba-Geigy and Sandoz, Novartis is a global corporation based out of Basel, Switzerland.[16] Over 155 countries worldwide have Novartis products available for use.[17] Financial data from 2016 reveals net sales of $48.5 billion for the Swiss company.[17]
Novartis divides its shares into two major market exchanges: the ordinary shares (NOVN SW) trade in the Six Swiss Exchange while the American Depositary Receipts (NVS US) trade in the New York Stock Exchange.[18] Nominees, fiduciaries, and ADR depositary make up the bulk of registered shareholders of Novartis stock while individual shareholders make up the lowest percentage.[19]
Originally launched in 2014, Zykadia sales for Fiscal Year 2016 reached $91 million.[20] While this is substantially less than several of their other pharmaceuticals, the new indication introduced in 2017 should result in increased sales of the drug. GlobalData predicts ceritinib sales to exceed $127million by 2025, while sustaining a compounded annual growth rate of 10.7%.[21]
Intellectual property
Novartis currently owns twelve patents on Zykadia.[22] The patents relate to different structures of the chemical compound as well as methodologies for manufacturing the drug. For example, one patent examines the structure of pyrimidines and their use in treatment of neoplastic diseases.[23] Others examine the composition of protein kinase inhibitors.[24] The most recent patents are specific the methodologies of using ALK inhibitors.[25]
See also
References
- "Zykadia (ceritinib) Capsules, for Oral Use. Full Prescribing Information" (PDF). Novartis Pharmaceuticals Corporation. Retrieved 14 February 2017.
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 71" (PDF). World Health Organization. 2014. p. 79. Retrieved 14 February 2017.
- "FDA Approves Ceritinib for ALK-Positive Lung Cancer". Medscape. April 29, 2014.
- "Prescribing data" (PDF). www.pharma.us.novartis.com. Retrieved 2019-06-12.
- "FDA Expands Ceritinib Approval for Lung Cancer". National Cancer Institute. 27 June 2017.
- "Analplastic lymphoma kinase (ALK) fusion oncogene positive non-small cell lung cancer". UpToDate. Wolters Kluwer. Retrieved 30 October 2017.
- "Roche announces FDA approval of companion diagnostic to identify ALK-positive non-small cell lung cancer patients". diagnostics.roche.com.
- Au T.H., Cavalieri C.C., Stenehjem D.D. Ceritinib: A primer for pharmacists // Oncology pharmacy practice. — 2017. — Vol. 23(8). — P. 611 (doi: 10.1177/1078155216672315)
- "Login". online.lexi.com.
- "Anapestic lymphoma kinase (ALK) fusion oncogene positive non-small cell lung cancer". UpToDate. Wolters Kluwer. Retrieved 30 October 2017.
- "ZYKADIA® (ceritinib) Dosing & Administration | HCP". www.hcp.novartis.com. Retrieved 2019-04-28.
- Khozin S, Blumenthal GM, Zhang L, Tang S, Brower M, Fox E, et al. (June 2015). "FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer". Clinical Cancer Research. 21 (11): 2436–9. doi:10.1158/1078-0432.CCR-14-3157. PMID 25754348.
- "Novartis drug Zykadia receives FDA Priority Review for first-line use in patients with ALK+ metastatic NSCLC". Novartis.
- Soria JC, Tan DS, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. (March 2017). "First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study". Lancet. 389 (10072): 917–929. doi:10.1016/s0140-6736(17)30123-x. PMID 28126333. S2CID 4739527.
- "Brochure" (PDF). www.us.zykadia.com. Retrieved 2019-06-12.
- "Novartis Company History". Novartis Global. Retrieved 3 November 2017.
- "Novartis Annual Reporting Suite". Novartis.
- "Share Overview". Novartis.
- "Share Ownership". Novartis.
- "Interim financial report" (PDF). www.novartis.com. 2017. Retrieved 2019-06-12.
- Xuan C, Gunduz V. "NSCLC MARKET – Global Drug Forecast & Market Analysis to 2025". Drug Development & Delivery. No. November–December 2016.
- "Zykadia - Patents - Expiry - Expiration - Dates". PharmaCompass.com.
- "US Patent No.: 7964592" (PDF). PharmaCompass. Retrieved 1 November 2017.
- "US No.: 8399450". PharmaCompass. Retrieved 1 November 2017.
- "US No.: 8703787". PharmaCompass. Retrieved 1 November 2017.
