Avapritinib
Avapritinib, sold under the brand name Ayvakit among others, is a medication used for the treatment of tumors due to one specific rare mutation: it is specifically intended for adults with unresectable or metastatic gastrointestinal stromal tumor (GIST) that harbor a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation.[1][3]
 | |
| Clinical data | |
|---|---|
| Pronunciation | A va PRI ti nib |
| Trade names | Ayvakit, Ayvakyt |
| Other names | BLU-285, BLU285 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620013 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Antineoplastic agents |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
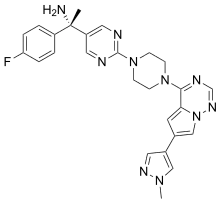
| Formula | C26H27FN10 |
| Molar mass | 498.570 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects are edema (swelling), nausea, fatigue/asthenia (abnormal physical weakness or lack of energy), cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation (secretion of tears), abdominal pain, constipation, rash and dizziness.[3]
Avapritinib is a kinase inhibitor.[3]
Medical uses
Ayvakyt is indicated as monotherapy for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) harboring the platelet-derived growth factor receptor alpha (PDGFRA) D842V mutation.
History
The U.S. Food and Drug Administration (FDA) approved avapritinib in January 2020.[3][4] The application for avapritinib was granted fast track designation, breakthrough therapy designation, and orphan drug designation.[3] The FDA granted approval of Ayvakit to Blueprint Medicines Corporation.[3]
Avapritinib was approved based on the results from the Phase I NAVIGATOR[5][6] clinical trial involving 43 subjects with GIST harboring a PDGFRA exon 18 mutation, including 38 subjects with PDGFRA D842V mutation.[3] Subjects received avapritinib 300 mg or 400 mg orally once daily until disease progression or they experienced unacceptable toxicity.[3] The recommended dose was determined to be 300 mg once daily.[3] The trial measured how many subjects experienced complete or partial shrinkage (by a certain amount) of their tumors during treatment (overall response rate).[3] For subjects harboring a PDGFRA exon 18 mutation, the overall response rate was 84%, with 7% having a complete response and 77% having a partial response.[3] For the subgroup of subjects with PDGFRA D842V mutations, the overall response rate was 89%, with 8% having a complete response and 82% having a partial response.[3] While the median duration of response was not reached, 61% of the responding subjects with exon 18 mutations had a response lasting six months or longer (31% of subjects with an ongoing response were followed for less than six months).[3]
The FDA approved avapritinib based on evidence from one clinical trial (NCT02508532) of 204 subjects with GIST.[4] The trial was conducted at 17 sites in the United States, Europe and Asia.[4]
Avapritinib showed a median PFS of 4.2 months compared to 5.6 months for regorafenib. The difference in median PFS between the avapritinib and regorafenib groups was not statistically significant. The overall response rate was 17 percent for the avapritinib group and 7 percent for the regorafenib group. The VOYAGER trial evaluated the efficacy and safety of avapritinib (N=240) versus regorafenib (N=236) in patients with third- or fourth-line GIST.[7] Based on the top-line VOYAGER data, the company plans to discontinue further development of avapritinib in GIST beyond PDGFRA exon 18 mutant GIST.
Avapritinib was approved for medical use in the European Union in September 2020.[2]
References
- "Ayvakit- avapritinib tablet, film coated". DailyMed. 21 January 2020. Retrieved 29 September 2020.
- "Ayvakyt EPAR". European Medicines Agency (EMA). 20 July 2020. Retrieved 29 September 2020.
- "FDA approves the first targeted therapy to treat a rare mutation in patients with gastrointestinal stromal tumors". U.S. Food and Drug Administration (FDA) (Press release). 9 January 2020. Archived from the original on 11 January 2020. Retrieved 9 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Drug Trial Snapshot: Ayvakit". U.S. Food and Drug Administration (FDA). 9 January 2020. Retrieved 24 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Blueprint Medicines Announces FDA Approval of Ayvakit (avapritinib) for the Treatment of Adults with Unresectable or Metastatic PDGFRA Exon 18 Mutant Gastrointestinal Stromal Tumor". Blueprint Medicines Corporation (Press release). 9 January 2020. Archived from the original on 11 January 2020. Retrieved 9 January 2020.
- "Blueprint Medicines Announces Updated NAVIGATOR Trial Results in Patients with Advanced Gastrointestinal Stromal Tumors Supporting Development of Avapritinib Across All Lines of Therapy". Blueprint Medicines Corporation (Press release). 15 November 2018. Archived from the original on 10 January 2020. Retrieved 9 January 2020.
- "Blueprint Medicines Announces Top-line Results from Phase 3 VOYAGER Trial of Avapritinib versus Regorafenib in Patients with Advanced Gastrointestinal Stromal Tumor". Blueprint Medicines Corp. Retrieved 28 April 2020.
Further reading
- Wu CP, Lusvarghi S, Wang JC, et al. (July 2019). "Avapritinib: A Selective Inhibitor of KIT and PDGFRα that Reverses ABCB1 and ABCG2-Mediated Multidrug Resistance in Cancer Cell Lines". Mol. Pharm. 16 (7): 3040–3052. doi:10.1021/acs.molpharmaceut.9b00274. PMC 6620786. PMID 31117741.
- Gebreyohannes YK, Wozniak A, Zhai ME, et al. (January 2019). "Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-derived Xenograft Models of Gastrointestinal Stromal Tumors". Clin. Cancer Res. 25 (2): 609–618. doi:10.1158/1078-0432.CCR-18-1858. PMID 30274985.
External links
- "Avapritinib". Drug Information Portal. U.S. National Library of Medicine (NLM).
- "Avapritinib". NCI Dictionary of Cancer Terms. National Cancer Institute.
- "Avapritinib". National Cancer Institute.
